The recent increase in antibiotic resistance has invoked the need for an alternative strategy to cure microbial infection. Bacteriophages could be used as an alternative therapy for treating multidrug-resistant bacterial infections in animals and humans.
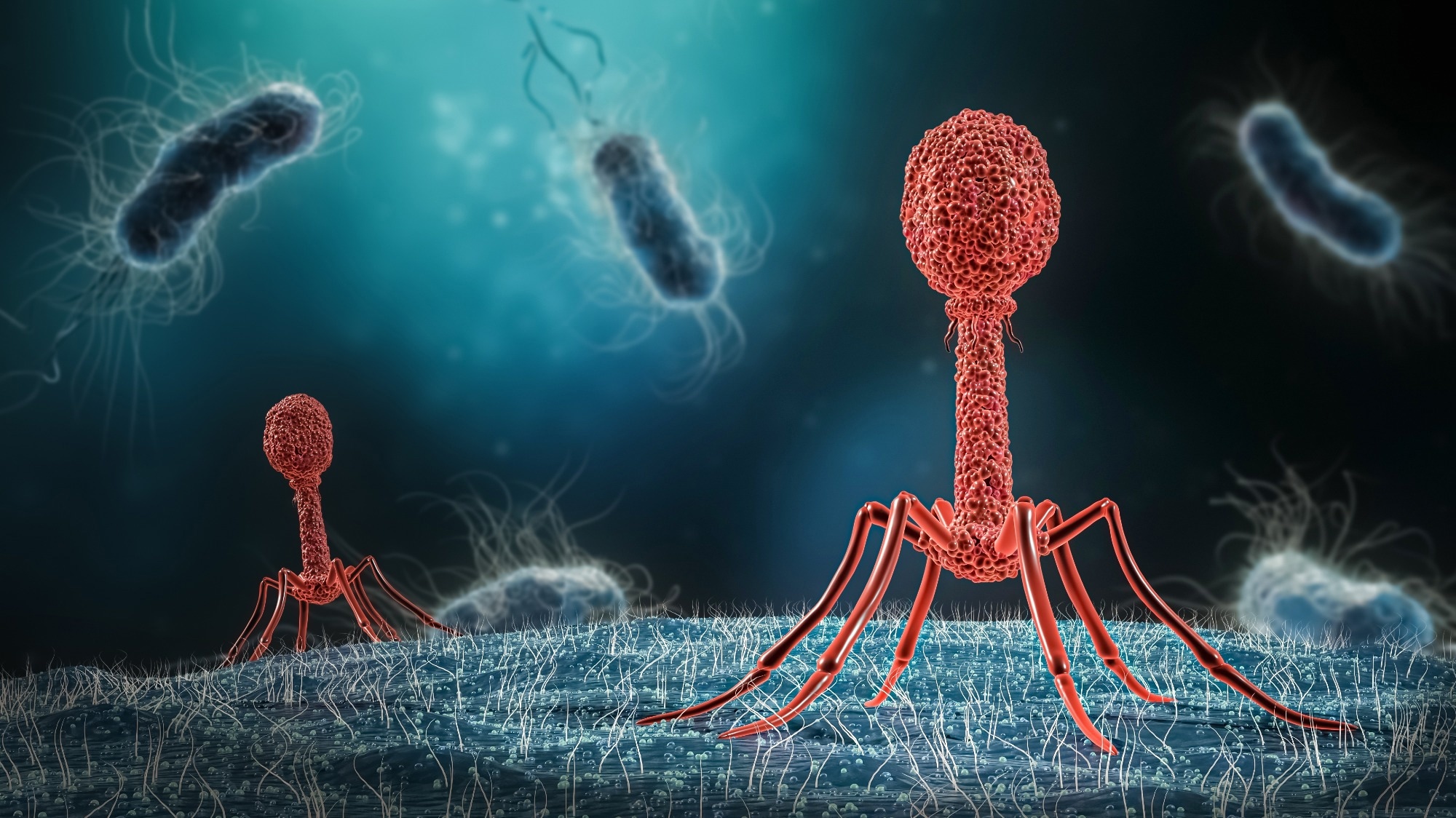 Image Credit: MattL_Images/Shutterstock.com
Image Credit: MattL_Images/Shutterstock.com
Understanding the Potential of Bacteriophages as a Therapeutic Tool
Bacteriophages, also known as phages, were discovered early in the 20th century.1 These are viruses that can infect and replicate within different bacteria; however, they cannot infect eukaryotic cells.
Similar to any viruses, bacteriophages rely on the host molecular machinery for replication and proliferation. In some cases, the metabolic processes of the host bacteria get completely disrupted after being infected by bacteriophages. These metabolic changes lead to lysis and bacterial death.2
Although the therapeutic potential of bacteriophages was identified soon after their discovery, their application in clinical settings was compromised due to logistic challenges in research. As some of the earlier research on bacteriophages failed to reflect upon their statistically significant efficacy against bacteria, the motivation to continue exploring these organisms was low.
Simultaneously, the scalable outcomes of antibiotic research increased their popularity for the treatment of bacterial infections. Soon, antibiotics monopolized the market and attracted huge investment from large pharmaceutical companies.3
Despite the earlier dip in interest in bacteriophages, scientists quickly realized their true potential in scientific research. Phages were used to uncover many fundamental aspects of molecular biology, such as horizontal gene transfer.4
The scientific community has recently regained interest in exploring their potential in clinical settings because bacteriophages are an important driver of bacterial evolution and are highly host-specific.
The Growing Crisis of Antibiotic Resistance
Antibiotics are a group of medicines that are used to treat bacterial infections.5 However, the widespread overuse and misuse of antibiotics, and the lack of new antibiotics have led to the development of antibiotic resistance in many pathogens.
Antibiotic resistance is a formidable threat to modern medicine because multiple treatments, such as cancer therapy and organ transplantation, significantly rely on the efficacy of antibiotics to control potential bacterial infections.6
Many organizations, such as the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), the Infectious Diseases Society of America, and the World Economic Forum, have declared antibiotic resistance to be “a global public health concern”.7
At present, scientists across the world are fighting to develop effective strategies to curb the problem of antibiotic resistance. So far, global antibiotic resistance displayed no signs of decline. Perhaps there is a need for a globally coordinated campaign to delineate the impact of antibiotic resistance.
Some bacterial species that exhibited a higher threat to antibiotic effectiveness are Staphylococcus aureus, Enterococcus faecium, Enterobacter faecium, Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Other bacteria that exhibited antibiotic resistance are Shigella, Salmonella, and Escherichia coli.8
Mechanisms of Bacteriophage Action Against Bacteria
Mechanistically, bacteriophage binds to a specific host, infects, and subsequently follows either one of the two replication processes, namely, lytic or lysogenic cycle.9 During the lytic replication process, the bacteriophage attaches to the susceptible host and introduces its genomic component to the host cell cytoplasm.
Here, the host’s ribosomes and molecular machinery are used to manufacture multiple copies of the original bacteriophage. Subsequently, the host cell dies which are either actively or passively lysed to release new bacteriophages to infect other susceptible host cells.
Similar to the lytic cycle, the lysogenic cycle is also associated with phage attaching to the host and transferring its genome to the host cytoplasm. However, in the lysogenic cycle, the bacteriophage’s genome gets integrated into the bacterial chromosome.
Therefore, the phage genome is transferred to the newly developed sister bacterial cells after bacterial replication. The bacterial cells containing the phage genome are known as lysogens. These cells can trigger lysis and kill host cells in response to changing environmental conditions.
T4 Phage attacking E.coli
Bacteriophage Therapy to Mitigate Antibiotic Resistance
The use of bacteriophages as antibacterial agents is referred to as bacteriophage or phage therapy. Mechanistically, this therapy entails binding of bacteriophage to susceptible bacteria and induction of rapid lysis to kill them.10 With the prevalence of the global threat of antibiotic resistance, bacteriophage therapy could be used as a safe and alternative approach to alleviate bacterial infection
In comparison to traditional antibiotic therapies, bacteriophage therapies have additional benefits. Experimental studies have shown bacteriophage therapy's effectiveness against Gram-positive and Gram-negative pathogenic bacteria.
Since bacteriophages are abundantly found in the soil, water, hot springs, hospital effluent, and human/animal gastrointestinal tract, these can be easily isolated. This ease in isolation techniques can significantly reduce the development cost compared to antibiotics formulation. Importantly, bacteriophages have exhibited reduced side effects due to their ubiquitous presence and constant contact with humans.2
Technological advancements have enabled the use of bacteriophages as diagnostic tools to detect bacterial pathogens. Phage therapy can decrease the leukocyte counts and mean C-reactive protein (CRP) values. In contrast to the use of antibiotics that reduce normal flora along with pathogens, bacteriophage therapy is highly specific and does not harm beneficial bacterial strains.11
Bacteriophages have the tendency to proliferate at the infection site, mainly due to their innate self-replicating property. This is extremely beneficial because the persistent presence of phages is expected to prevent the potential growth of secondary pathogens. This phenomenon entails lowering the need for higher or multiple bacteriophage dosage to cure bacterial infection.
The fast distribution of bacteriophages in different organs, including bones, brains, and prostate glands, which are not readily accessible by multiple drugs, make bacteriophages a potential tool to cure bacterial infection. The absence of cross-resistance to antibiotics is another advantage of bacteriophages because it prevents interference with their effectiveness against bacteria.12 The use of bacteriophage cocktails has displayed greater efficacy than using a single bacteriophage to prevent or treat bacterial diseases.11
At present, bacteriophage therapies have been used to treat multiple bacterial infections caused by P. aeruginosa, S. aureus, E. faecalis, and A. baumannii. The efficacy of this treatment could be explained using a recent bacterial infection case.
A 68-year-old diabetic patient who developed necrotizing pancreatitis along with multiple drug-resistant A. baumannii infection was treated with nine different lytic phages. Here, bacteriophages were introduced percutaneously and intravenously into the abscess. Notably, this treatment effectively cleared the infection and significantly improved the patient’s condition.13
Sources
- Keen EC. A century of phage research: bacteriophages and the shaping of modern biology. Bioessays. 2015;37(1):6-9.
- Podlacha M, Grabowski Ł, Kosznik-Kawśnicka K, et al. Interactions of Bacteriophages with Animal and Human Organisms-Safety Issues in the Light of Phage Therapy. Int J Mol Sci. 2021;22(16):8937
- Simpkin VL, Renwick MJ, Kelly R, Mossialos E. Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps. J Antibiot (Tokyo). 2017;70(12):1087-1096.
- Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage. 2011;1(1):31-45.
- Adedeji WA. THE TREASURE CALLED ANTIBIOTICS. Ann Ib Postgrad Med. 2016;14(2):56-57.
- Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277-283.
- Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-1658.
- Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162-173.
- Hitchcock NM, Devequi Gomes Nunes D, Shiach J, et al. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses. 2023;15(4):1020.
- Liu C, Hong Q, Chang RYK, Kwok PCL, Chan H-K. Phage–Antibiotic Therapy as a Promising Strategy to Combat Multidrug-Resistant Infections and to Enhance Antimicrobial Efficiency. Antibiotics. 2022; 11(5):570.
- Taati Moghadam M, Amirmozafari N, Shariati A, et al. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect Drug Resist. 2020;13:45-61.
- Hetta HF, Rashed ZI, Ramadan YN, Al-Kadmy IMS, Kassem SM, Ata HS, Nageeb WM. Phage Therapy, a Salvage Treatment for Multidrug-Resistant Bacteria Causing Infective Endocarditis. Biomedicines. 2023; 11(10):2860.
- Schooley RT, Biswas B, Gill JJ, et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection [published correction appears in Antimicrob Agents Chemother. 2018 Nov 26;62(12):]. Antimicrob Agents Chemother. 2017;61(10):e00954-17.
Further Reading