Dr. Manel Esteller speaks to AZoLifeSciences about his ground-breaking cancer research in which he uncovered the behavior of DNA in 3D models.
What provoked your research into the behavior of cancer cells?
We know a lot about cancer from two models: the primary tumors extracted from surgical procedures, and cancer cells that grow in the lab within flasks called cell cultures.
These systems both have problems. Primary tumors have a limited amount of biological material and cannot be propagated, whereas the cell lines divide in an unnatural environment and only in 2D.
That is the reason we decided to study cancer cells because if there was a third option that recapitulated the actual tumors that could lead us to assay the value of these cancer structures called organoids.
Image Credit: crystal light/Shutterstock.com
What is an epigenetic fingerprint?
All our cells have the same genetic setting: our gut and brain look the same at the DNA sequence level. However, these tissues have very different functions. How is this possible?
The explanation that resides is that each tissue has a different “software”, a profile of chemical marks that control the right expression of the right gene at the right time. That regulatory system is called epigenetics (literally meaning features that are “on top” of genetics).
Cancer cells have aberrant epigenetic software that induces proliferation and metastasis.
Thus, the epigenetic fingerprint is the collection of the chemical modifications that control the switching on and off of our genes.
What is an organoid?
The organoid is established from a tumoral piece derived from the removal of a human tumor in the surgery room.
This biological material is then disaggregated in isolated cells, that with the right culture conditions, grow in three dimensions, like the original tumor the cells came from.
Thus, an organoid is a cancer grown in 3D cell culture and it can be studied for a long time.
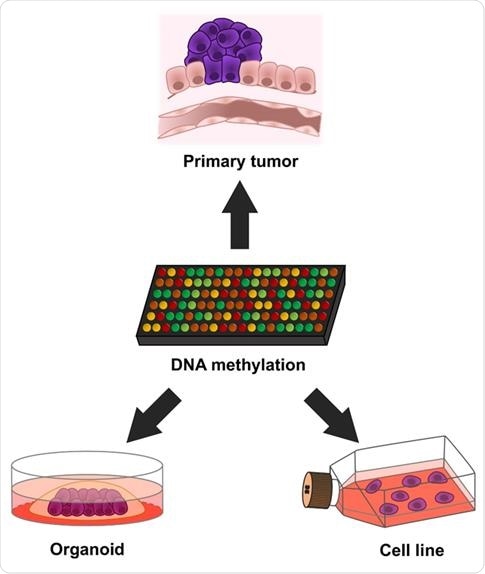
Image Credit: Manel Esteller
How did you use 3D models to develop a characterization of DNA make-up?
We used DNA extracted from these cancer cells grown in 3D to determine the patterns of chemical modifications occurring in the DNA molecule.
Our DNA is a mixture of four bases A + T + C + G, and we particularly focused on DNA methylation, which is the addition of a methyl group in the C bases.
This modification acts as a “stop signal”, blocking the expression of the underlying gene. We studied close to one million sites for DNA methylation using genomic and bioinformatic approaches.
Why do so many promising cancer therapies currently fail when applied to clinical settings despite showing promise in the lab? How could organoids overcome these issues?
There are many reasons. Among these, is the fact that most of these drugs first showed efficacy in 2D cancer models in the preclinical labs, whereas the actual tumors are 3D.
The use of 3D organoids can help to solve that problem.
Image Credit: Hakat/Shutterstock.com
You found that the properties of the organoid cancer cells in your research were notable. Why were they interesting?
There were three interesting results: the organoids mimicked the cancer types of the cancer specimen obtained from surgery, i.e. the colorectal cancer organoid looked like a colorectal cancer biopsy; the cancer organoids lacked contaminating normal cells, thus the malignant cells are easy to study; and finally, the cancer organoids resembled the surgical specimens more than the 2D long-established cancer cell cultures.
How will this research benefit different scientific communities in researching and providing drugs and treatment for cancer? What sort of oncology treatments could be revolutionized as a result?
The study proves that organoids are valuable tools in studying the effect of all drugs by targeting cancer cells themselves, rather than the surrounding environment.
They can be tested for tyrosine-kinase inhibitors or DNA damaging agents, for example. The effect can be easily obtained by determining cell growth inhibition or cell death.
This approach speeds up preclinical drug development and increases the chance of success in the posterior clinical trials.
How have you validated your research and how are you promoting collaboration and encouraging further breakthroughs in cancer research?
All the obtained epigenetic data of the studied organoids have been deposited in public databases for additional data mining and validation.
In addition, the assessed cancer organoids are available from a widely use provider (American Type Culture Collection) and every biomedical researcher can further interrogate them to obtain more interesting data.
What are the next steps in your research?
We would love to extend our study of the epigenetic landscape of human cancer organoids to other tumor types not initially included in the study, such as breast and brain cancer.
We would also like to study how the epigenetic blueprints of cancer organoids change if they are co-cultured with immune cells, another exciting area in cancer research.
Image Credit: Kateryna Kon/Shutterstock.com
Where can readers find more information?
The results of our research are described in an article in the Taylor & Francis journal “Epigenetics” (http://dx.doi.org/10.1080/15592294.2020.1762398). All the raw data can be downloaded from the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (NCBI) under accession number GSE144213.
About Dr. Manel Esteller
Dr. Manel Esteller graduated in Medicine from the Universität de Barcelona, where he also obtained his Ph.D. in molecular genetics.
Dr. Esteller was a Postdoctoral Fellow and a Research Associate at Johns Hopkins where he studied DNA methylation and human cancer. His work was decisive in establishing promoter hypermethylation of tumor suppressor genes as a common hallmark of cancer.
From October 2001 to September 2008 Manel Esteller was the Leader of the CNIO Cancer Epigenetics Laboratory, where his principal area of research was the alterations in DNA methylation, histone modifications, and chromatin in human cancer.
Since October 2008 until May 2019, Dr. Esteller has been the Director of the Cancer Epigenetics and Biology Program (PEBC) of the Bellvitge Institute for Biomedical Research (IDIBELL) in Barcelona.
He is currently the Director of the Josep Carreras Leukaemia Research Institute (IJC), Chairman of Genetics in the School of Medicine of the University of Barcelona, and an ICREA Research Professor.
His current research is devoted to the establishment of the epigenome maps in health and disease, and the development of new epigenetic drugs.
He is the author of many highly cited peer-reviewed manuscripts in biomedical sciences, he is also a member of numerous international scientific societies, Editorial Boards and reviewer for many journals and funding agencies.
He has received prestigious recognition for his scientific achievements among them the World Health Summit Award, the Swiss Bridge Cancer Award, and the EACR Cancer Researcher Award Lecture.