In this interview, we speak to Professor Kelly Schultz and colleagues about their latest research that has led to the development of a new lab-grown meat that could help overcome challenges faced by current techniques.
Please can you introduce yourself and tell us what inspired your latest research into lab-grown meat?
My name is Kelly Schultz and I am an associate professor of Chemical and Biomolecular Engineering at Lehigh University. My lab focuses on the characterization of gels, ranging from gels used in fabric and home care products, to those used as scaffolds for tissue engineering.
Our latest research into lab-grown meat is a team effort comprising of myself, Mark Snyder, Angela Brown, and Steve McIntosh, who are all colleagues of mine in Chemical and Biomolecular Engineering at Lehigh University. We decided we wanted to develop a project together and I had been thinking about cultured meat for a while.
The reason I had been thinking about cultured meat is because it is a tissue engineering program, just with animal instead of human cells. In conversations with my collaborators, we determined that some of the hurdles that are commonly faced with cultured meat could be tackled by our unique set of skills and that we had a new and novel approach to see if we could grow whole-cut meat products.
Meat production is responsible for over half of global greenhouse gas emissions. Why would you say this is, and why is it so important to find new sustainable methods for the production of meat and food?
Many factors contributing to meat production contribute to greenhouse gas emissions. Some great papers cover this. A lot of it is the cattle, fertilizer, water usage, waste, etc. Since the meat market keeps growing it is really not feasible to continue to increase factory farming.
I think it should also be noted that factory farming is the main contributor to this problem. So there is definitely a market for cultured meat to reduce the environmental effects of factory farming and still provide the meat that is needed.

Image Credit: El Nariz/Shutterstock.com
Please can you describe how cultured meat is produced? Are there any limitations surrounding current production methods? If so, what are they?
To be commercially viable, a process for manufacturing cultured meat must satisfy three essential criteria: (i) appropriate taste and texture, (ii) large enough tissue constructs to serve as consumable meat, and (iii) cost-effective manufacturing. While there are approximately 30 established cultured meat companies, no company has successfully brought a product to market.
Several cultured meat companies claim to be near commercialization, but their specific methods for growing meat remain trade secrets, and the quality and economic viability of their products are unproven.
At present, two methods for producing cultured meat have been studied; suspension culture on microcarriers in a CSTR, and cultures within scaffolds in a perfusion reactor. While the suspension culture technique has received the most attention, it is severely limited in the size and organization of the resulting muscle fibers grown on the microcarriers.
Microcarriers are used because muscle cells require adhesion to proliferate, yet microcarrier separation from the culture is difficult and an additional processing step. In contrast, the use of scaffolds in perfusion reactors has the potential to produce large muscle constructs and make cultured meat manufacturing economical and viable, but strategies are needed to overcome limits on dissolved oxygen and nutrient transport, cost of chemical cues provided in the culture, waste removal, and bioreactor size.
The taste of cultured meat mostly mimics the animal from which cells are taken, but the lack of order and organization leads to the soft texture that is reminiscent of ground meat. Achieving textures consistent with meat produced from whole animals requires the organization of myofibers in the cultured meat sample and the development of substructure induced by exercise. Recent studies aiming to increase the size and organization of tissue have employed soy-based scaffolds, but these impact taste upon cooking.
Cultured meat also suffers from high cost and small product yields due to the use of large amounts of expensive growth factors constantly replenished in the culture media required to provide chemical cues that encourage muscle tissue development.
In addition, many governance issues are expected to emerge once products are commercialized. For example, the issue of labeling and naming of the product remains unclear and based on previous experience with another novel food, genetically engineered crops, these debates could take decades to resolve. The proposed research will explore these regulatory challenges to identify potential barriers to the translation of the developed technology.
How did your latest research overcome some of these limitations? How is your cultured meat developed?
The project goal is to realize a transformative tissue engineering strategy using a novel engineered composite scaffold that provides physical and spatially targeted chemical cues to grow and facilitate the organization of muscle tissue into a whole-cut meat product (Figure 1) at a reasonable cost. This scaffold will be a temporally evolving photopolymerized poly(ethylene glycol) (PEG)-peptide hydrogel nanocomposite embedded in an anisotropic macroporous collagen-mimic (e.g., synthetic collagen matrix, polysaccharide networks) that simulates aspects of the skeletal muscle microenvironment (Aim 1).
The hydrogel scaffold will locally present physical and chemical cues necessary to control encapsulated cell lineage specification. Hydrogel-implanted lipid-based vesicles will provide on-demand oxygen and nutrients throughout the 3D structure, avoiding the complexity of artificial vasculature (Aim 2). Nervous system-derived cues necessary for the organization and development of muscle fibers will be replicated using electrochemical stimulation (Aim 3).
Together, this highly tunable scaffold, novel mode of local oxygen and nutrient delivery, and integrated electrochemical stimulation offers a transformative, scalable, and cost-effective approach to 3D meat cultivation that can be adapted for a range of animal cells while reducing waste.
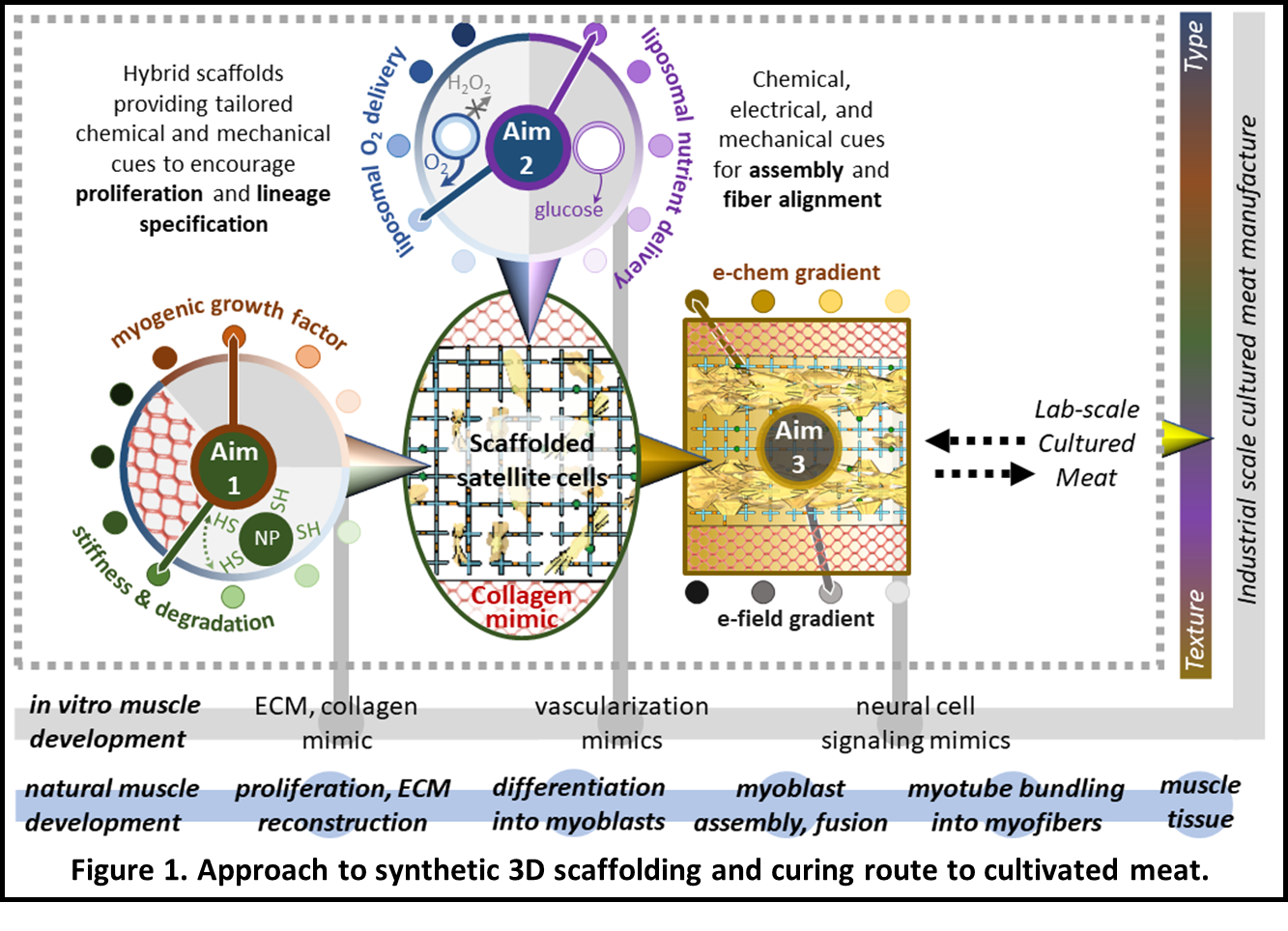
Figure 1
Your research team consisted of chemical engineers from a diverse array of research areas. How did this versatility benefit your research?
As mentioned in the development of this project, it was really our unique skills that brought this idea together. I work on the characterization of tissue engineering scaffolds for humans, so it was an obvious fit for me to just change the cells we were focusing on to animal cells and start to work on the design of the scaffold to fit the needs of the growth of animal muscle tissue.
Snyder is an expert in materials chemistry toward scaffold hybridization and in this project, his expertise will enable the structuring of the tissue engineering scaffold for controlling cell proliferation, lineage specification, migration, and assembly.
Brown brings expertise in biological colloid science and will develop liposomes for oxygen and nutrient delivery, replacing the need for vasculature within the tissue culture platform. McIntosh brings expertise in electrochemistry and material science to address directed cellular growth under electrochemical gradients and electric fields as a replacement for electrical signaling, for stimulating and directing muscle fiber growth and texture (as he puts it “exercising the meat”).
With heightened awareness surrounding climate change and sustainability, do you think we will see more people turning to alternative meat solutions and more companies venturing into this space?
There are already a lot of companies working on aspects of cultured meat and working in this space already. I think it is really an emerging area and we will see more people turning their attention to alternative meat solutions.
As part of our funding from the Good Food Institute, we were able to attend their conference this year and it was really eye-opening to see how many people are working in this space and the excitement around these types of emerging products. It is really my hope that alternative meats become a common product and that science enables us to develop new technologies for the growth of cultured meat products.
With continued research, are you hopeful that your cultured meat could one day be available in supermarkets? What steps are needed before this can become a reality?
I hope that cultured meat is a product available to everyone. I think the biggest hurdle at the moment is the price of cultured meat and the size and texture of the product that is being produced. These are all aspects of cultured meat that are being tackled and hopefully, everything will come together to enable this product to appear in our grocery stores.
What does the future of the meat industry look like to you?
I hope that the meat industry moves away from factory farming and more to small farming and alternative meat products. This will provide the products that people want and also tackle the damage that factory farming does.

Image Credit: Firn/Shutterstock.com
What are the next steps for you and your research into cultured meat?
We are just starting our project and are very excited about getting this work done. At present, we are developing our composite scaffold to grow animal muscle cells and working on a liposome-based delivery system for oxygen and nutrient delivery to cells that will be incorporated into our synthetic scaffold.
Where can readers find more information?
Here are our faculty webpages and our research websites are linked on these pages
About Professor Kelly Schultz
Dr. Kelly M. Schultz is an Associate Professor in the Department of Chemical and Biomolecular Engineering at Lehigh University. She obtained her B.S. in Chemical Engineering from Northeastern University in 2006 and a Ph.D. in Chemical Engineering with Professor Eric Furst from the University of Delaware in 2011 as a National Science Foundation graduate research fellow. While at Delaware, she was invited to speak in the American Chemical Society Excellence in Graduate Polymers Research Symposium and was selected as the Fraser and Shirley Russell Teaching Fellow.
Following her Ph.D., she was a Howard Hughes Medical Institute postdoctoral research associate at the University of Colorado at Boulder working in the laboratory of Professor Kristi Anseth. As a postdoc, she was invited to participate in the Distinguished Young Scholars Summer Seminar Series at the University of Washington. She began her position as Assistant Professor at Lehigh University in 2013 and was named a P.C. Rossin Assistant Professor in 2016 – 2018.
Dr. Schultz was named one of TA Instruments Distinguished Young Rheologists (2014), was awarded an NSF CAREER award (2018), the Lehigh University Libsch Early Career Research Award (2019), and the P.C. Rossin College of Engineering and Applied Science Excellence in Research Scholarship & Leadership (2020).
Dr. Schultz and her research group study emerging hydrogel materials developed for biological applications, such as wound healing and tissue regeneration. Of particular interest is the development of bulk and microrheological techniques that measure how 3D encapsulated human mesenchymal stem cells degrade and remodel synthetic hydrogel scaffolds during motility.