Scientists at the University of Bergen (UiB) have discovered that proteins employ a shared chemical marker as a protective shield against degradation, thereby influencing their mobility and the aging process.
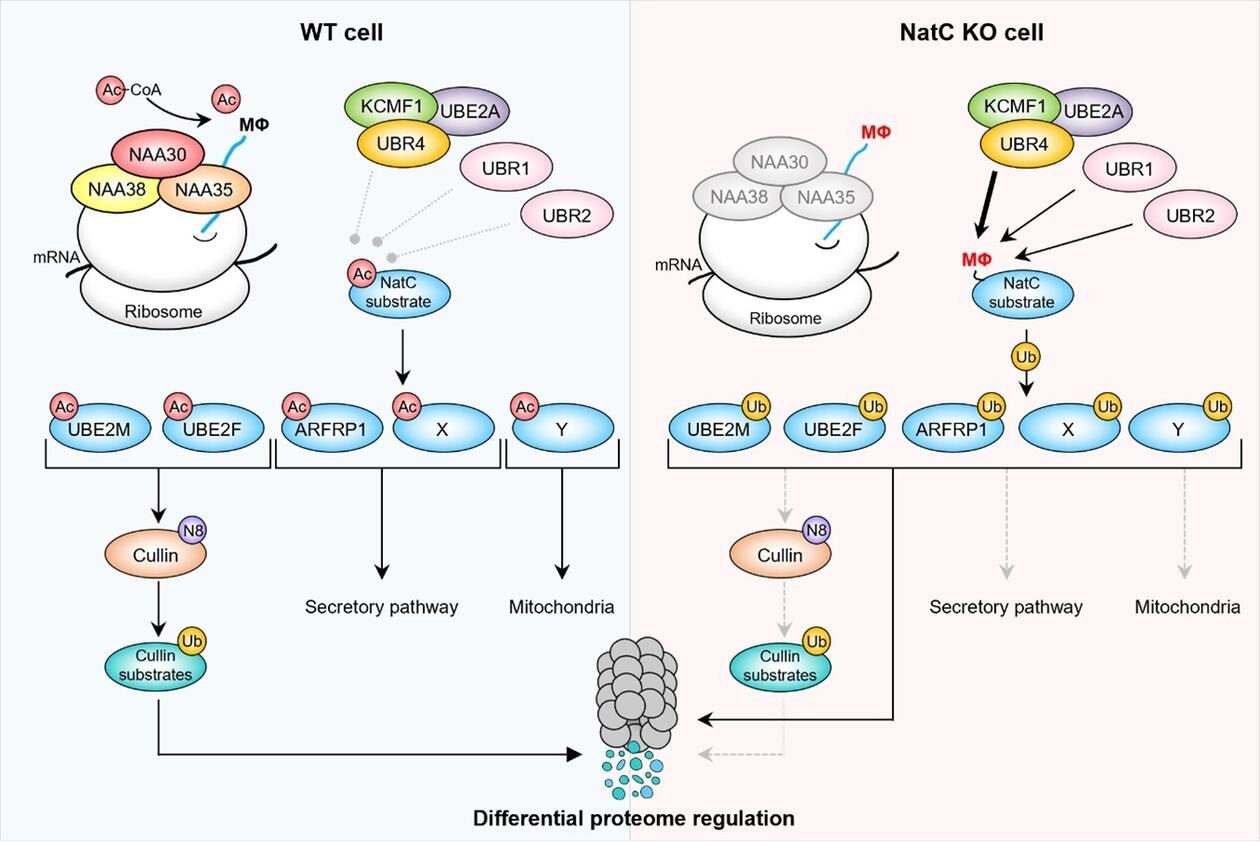 N-terminal acetylation by NatC shields proteins from degradation. (Left) The NatC complex acetylates proteins harboring a hydrophobic residue in the second position (MΦ). Following Nt-acetylation, Ac-UBE2M and Ac-UBE2F promote cullin neddylation (N8), resulting in ubiquitylation (Ub) and proteasomal degradation of targeted cullin substrates, Ac-ARFRP1 is targeted to the Golgi where it plays a role in the secretory pathway, while the hypothetical proteins Ac-X and Ac-Y are thought to affect the secretory pathway and mitochondria, respectively. (Right) Loss of NatC exposes unacetylated MΦ-starting N-termini which serve as N-degrons that can be recognized by a set of N-recognins leading to proteasomal and, in some cases, lysosomal degradation. Non-Nt-acetylated NatC substrates are primarily targeted by the ubiquitin ligases UBR4-KCMF1 and to some extent UBR1 and UBR2. Targeted degradation of non-Nt-acetylated NatC substrates leads to decreased cullin neddylation, increased mitochondrial elongation, and fragmentation, and is thought to affect intracellular trafficking. (Figure from Varland, Sylvia et al, 2023, Nature Communications) Image Credit: Varland, m.fl.
N-terminal acetylation by NatC shields proteins from degradation. (Left) The NatC complex acetylates proteins harboring a hydrophobic residue in the second position (MΦ). Following Nt-acetylation, Ac-UBE2M and Ac-UBE2F promote cullin neddylation (N8), resulting in ubiquitylation (Ub) and proteasomal degradation of targeted cullin substrates, Ac-ARFRP1 is targeted to the Golgi where it plays a role in the secretory pathway, while the hypothetical proteins Ac-X and Ac-Y are thought to affect the secretory pathway and mitochondria, respectively. (Right) Loss of NatC exposes unacetylated MΦ-starting N-termini which serve as N-degrons that can be recognized by a set of N-recognins leading to proteasomal and, in some cases, lysosomal degradation. Non-Nt-acetylated NatC substrates are primarily targeted by the ubiquitin ligases UBR4-KCMF1 and to some extent UBR1 and UBR2. Targeted degradation of non-Nt-acetylated NatC substrates leads to decreased cullin neddylation, increased mitochondrial elongation, and fragmentation, and is thought to affect intracellular trafficking. (Figure from Varland, Sylvia et al, 2023, Nature Communications) Image Credit: Varland, m.fl.
Proteins play a pivotal role in all cellular processes, and comprehending their functions and control mechanisms holds significant importance.
For many years, we have known that nearly all human proteins are modified by a specific chemical group, but its functional impact has remained undefined.”
Thomas Arnesen, Professor, Department of Biomedicine, University of Bergen
Arnesen added, “One of the most common protein modifications in human cells is N-terminal acetylation, which is an addition of a small chemical group (acetyl) at the starting tip (N-terminus) of a protein.”
“The modification is launched by a group of enzymes called N-terminal acetyltransferases (NATs)”. Despite being “everywhere” in human cells, the functional role of this modification remains mysterious,” continued Arnesen.
Arnesen is a researcher involved in a recent study that unveils a fundamental role of this protein modification, which is to safeguard proteins from deterioration, and this safeguard is crucial for maintaining typical lifespan and mobility.
CRISPR-Cas9 Technology Sheds New Light on N-Terminal Acetylation
In her quest for answers, molecular biologist and researcher Sylvia Varland dedicated two years to her work at the Donnelly Center for Cellular & Biomolecular Research at the University of Toronto in Canada. Her research was made possible through the support of a FRIPRO mobility grant from the Research Council of Norway.
During her time at this prestigious scientific institution, she harnessed the well-established CRISPR-Cas9 technology and state-of-the-art screening platforms. These resources allowed her to unravel the functional roles of the NAT enzymes in the human body.
Sylvia’s primary focus was on NatC, one of the principal NAT enzymes in humans. Through genome-wide screening of NatC knockout cells, she unearthed numerous human genes likely to be intricately involved in the mechanisms of N-terminal acetylation.
Without the inspiring scientific environment at the Donnelly Center combined with financial support from Marie Skłodowska-Curie Actions this study would not have seen the light of day.”
Sylvia Varland, Molecular Biologist and Researcher, University of Bergen
Upon returning to the Arnesen lab at UiB, Sylvia delved into the molecular ramifications of her genetic discoveries, collaborating with PhD student Ine Kjosås and fellow lab colleagues.
A series of biochemical, cell biology, and proteomics experiments shed light on the shielding effect of N-terminal acetylation, safeguarding numerous proteins from degradation. In contrast, proteins lacking N-terminal acetylation were identified and targeted by the cellular degradation machinery.
Varland added, “N-terminal acetylation has the power to dictate a protein’s lifetime and affects our cells in numerous ways. This is true for humans, and it is also true in fruit flies, which is a very useful model to study this protein modification.”
N-Terminal Acetylation Can Affect Aging
Simultaneously, an investigative team led by Rui Martinho at the University of Aveiro in Portugal embarked on an exploration of the organismal consequences of NatC-mediated N-terminal acetylation, employing a Drosophila fruit fly model.
Postdoctoral researcher Rui Silva and fellow students undertook investigations involving flies deficient in N-terminal acetylation. Afterward, the two research groups made a collective decision to join forces and, for the past two years, have been synchronizing their experimental endeavors.
Image Credit: Christoph Burgstedt/Shutterstock.com
The flies lacking NatC exhibited viability, but they demonstrated reduced longevity and decreased mobility as they aged. These effects could be partially mitigated by the expression of a protein that is conserved between flies and humans and has been identified as a critical target of NatC protection.
Decoding the NatC Puzzle
In summary, through an impartial and comprehensive genetic screening approach combined with cellular phenotyping, the research team unveiled a universal role for N-terminal acetylation in safeguarding proteins from degradation within human cells.
Their molecular inquiries pinpointed the cellular elements, specifically ubiquitin ligases, responsible for breaking down a significant class of human proteins when lacking N-terminal acetylation.
This protective function mediated by NatC is observable not only in human cells but also in fruit flies. The implications of these pathways on the lifespan and mobility of aging individuals underscore the critical significance of protein N-terminal acetylation.
“This work untangles some of the secrets and shows how N-terminal acetylation shape individual protein fate,” concluded Thomas Arnesen.
Source:
Journal reference:
Varland, S., et al. (2023) N-terminal acetylation shields proteins from degradation and promotes age-dependent motility and longevity. Nature Communications. doi.org/10.1038/s41467-023-42342-y.