Scientists at the University of Illinois Urbana-Champaign, in collaboration with researchers at the University of Wisconsin-Madison, have developed a novel antifungal molecule.
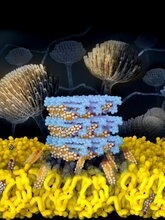 The mechanism for a critical but highly toxic antifungal is revealed in high resolution. Self-assembled Amphotericin B sponges (depicted in light blue) rapidly extract sterols (depicted in orange and white) from cells. This atomic level understanding yielded a novel kidney-sparing antifungal agent. Image Credit: Jose Vazquez
The mechanism for a critical but highly toxic antifungal is revealed in high resolution. Self-assembled Amphotericin B sponges (depicted in light blue) rapidly extract sterols (depicted in orange and white) from cells. This atomic level understanding yielded a novel kidney-sparing antifungal agent. Image Credit: Jose Vazquez
This new compound, created by modifying the structure of the well-known antifungal drug Amphotericin B, shows promise in utilizing the drug's effectiveness against fungal infections while mitigating its toxic effects.
The study has been reported in the journal Nature.
Amphotericin B, a naturally derived small molecule synthesized by bacteria, serves as a final option for combating fungal infections. Although highly effective in eradicating fungi, AmB is relegated to a last-resort status due to its toxicity to human patients, especially affecting the kidneys.
Fungal infections are a public health crisis that is only getting worse. And they have the potential, unfortunately, of breaking out and having an exponential impact, kind of like COVID-19 did. So let’s take one of the powerful tools that nature developed to combat fungi and turn it into a powerful ally.”
Dr Martin D. Burke, Research Leader and Professor, Chemistry, University of Illinois Urbana-Champaign
Burke holds a position as a professor at the Carle Illinois College of Medicine and is a practicing medical doctor. Over the years, Burke's team has dedicated extensive efforts to exploring Amphotericin B (AmB) with the goal of creating a derivative capable of eradicating fungi without causing harm to humans.
In earlier investigations, they employed a building block-based approach to molecular synthesis and collaborated with a group specializing in molecular imaging tools, led by Professor Chad Rienstra at the University of Wisconsin-Madison. Together, these teams unraveled the drug’s mechanism: AmB eliminates fungi by acting as a sponge, extracting ergosterol from fungal cells.
Image Credit: Vikks/Shutterstock.com
In their latest research, Burke’s team once again collaborated with Rienstra's group and discovered that AmB similarly targets and kills human kidney cells by extracting cholesterol, the most prevalent sterol in humans. The researchers also elucidated the atomic-level structure of AmB sponges when bound to both ergosterol and cholesterol.
The atomic resolution models were really the key to zoom in and identify these very subtle differences in binding interactions between AmB and each of these sterols.”
Corinne Soutar, Study Co-First Author and Graduate Student, University of Illinois Urbana-Champaign
Rienstra stated, “Using this structural information along with functional and computational studies, we achieved a significant breakthrough in understanding how AmB functions as a potent fungicidal drug. This provided the insights to modify AmB and tune its binding properties, reducing its interaction with cholesterol and thereby reducing the toxicity.”
Equipped with insights from the NMR studies, the Illinois team initiated the synthesis and testing of derivatives.
These variations involved subtle modifications to the region binding to ergosterol and cholesterol, along with enhancing the kinetics of the ergosterol-removal process to ensure sustained efficacy.
With support from collaborators and the resources at the Carl R. Woese Institute for Genomic Biology, alongside the expertise of Dr. Timothy Fan, a professor in veterinary clinical medicine at the University of Illinois, the researchers evaluated the most promising derivatives.
The evaluation began with in vitro assays, swiftly assessing their effectiveness in fungal eradication, then progressed to cell cultures, and ultimately to live mice to evaluate toxicity. Among the tested molecules, one named AM-2-19 emerged as particularly noteworthy.
This molecule is kidney-sparing, it is resistance evasive and it has broad spectrum efficacy. We tested this molecule against over 500 different clinically relevant pathogen species in four different locations. And this molecule completely surprised us by either mimicking or surpassing the efficacy of current clinically available antifungal drugs.”
Arun Maji, Study Co-First Author and Postdoctoral Researcher, University of Illinois Urbana Champaign
To evaluate potential toxicity, the researchers subjected AM-2-19 to testing in human blood and kidney cells. Additionally, they conducted trials using mouse models for three prevalent and resilient fungal infections, observing a notably high efficacy of AM-2-19 in these experiments.
Burke stated, “During my medical rotations, we called AmB ‘ampho-terrible,’ because of how hard it was on patients. Decoupling the efficacy from the toxicity turns ‘ampho-terrible’ into ‘ampho-terrific.’ We are very excited about the potential we are seeing, although clinical study is needed to see if this potential translates to people.”
In the initial step toward clinical implementation, AM-2-19 has been granted a license to Sfunga Therapeutics and is currently undergoing Phase 1 clinical trials. Sfunga Therapeutics played a supportive role in the research, contributing to the project’s funding. As a result, Burke received consulting income and equity in the company.
Source:
Journal reference:
Maji, A., et al. (2023) Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature. doi.org/10.1038/s41586-023-06710-4.