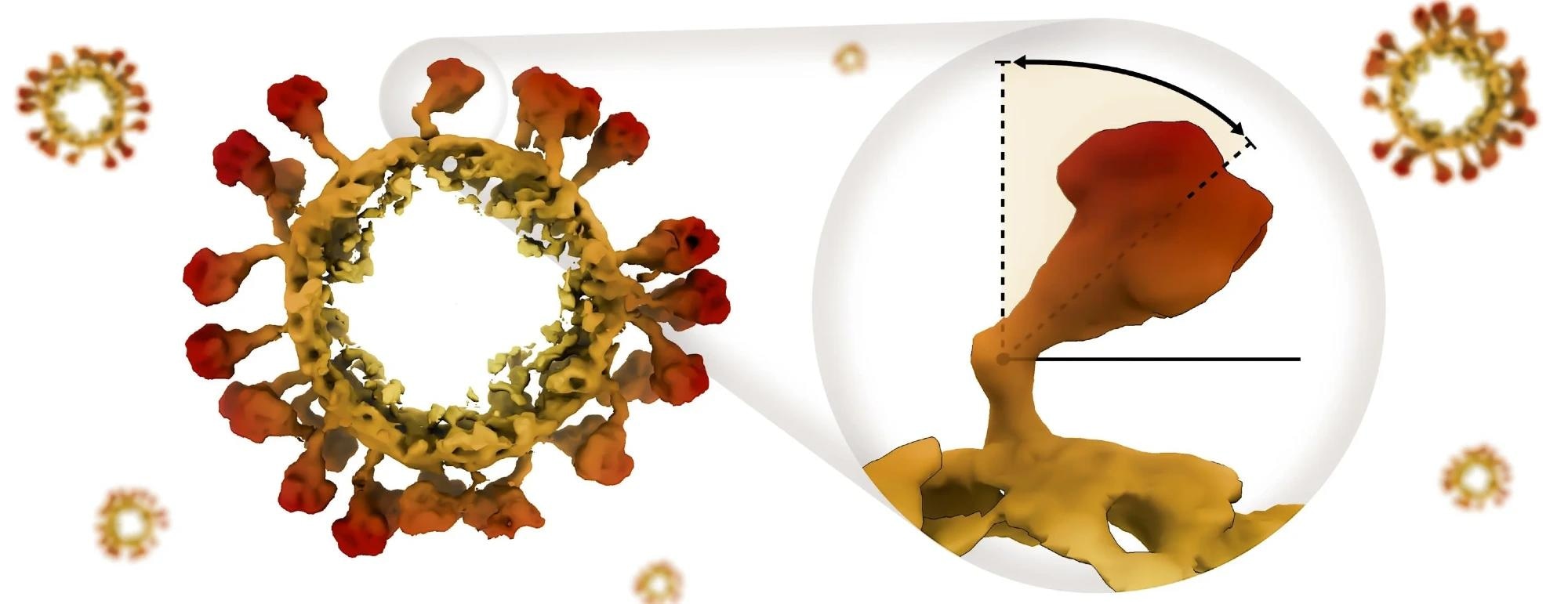
Coronaviruses employ protein “spikes” to latch onto and infect cells. Contrary to their name, these spikes do not have a rigid and pointed structure. Instead, they resemble chicken drumsticks, with the meaty portion facing outward.
 A study shows how the spikes of an infectious coronavirus bend on a tiny hinge, and how disabling those hinges could reduce infection. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory
A study shows how the spikes of an infectious coronavirus bend on a tiny hinge, and how disabling those hinges could reduce infection. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory
Importantly, the meaty part of these spikes has the flexibility to tilt in various directions along its slender stalk. This tilting capability plays a crucial role in determining the spike's effectiveness in infecting a cell.
Researchers from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University, in collaboration with three additional universities, have successfully captured high-resolution images of intact coronavirus spikes situated on virus particles.
Their findings include the identification of a minute hinge surrounded by sugar molecules, enabling the flexible bending of the spike’s globular “crown” along its stalk. Furthermore, the researchers have quantified the extent to which the spike can tilt in various directions.
The study focused on a less hazardous relative of SARS-CoV-2, the coronavirus responsible for COVID-19. Despite this difference, the research holds relevance for COVID-19. Both viruses are attached to the same receptor on the cell’s surface to initiate the infection process.
Jing Jin, a Biologist at Vitalant Research Institute and Adjunct Assistant Professor at the University of California, San Francisco, conducted virology experiments for the study and emphasized the implications of the findings for understanding and potentially addressing COVID-19.
She says, the results suggested that disabling the spike’s hinges could be a good way to prevent or treat a wide range of coronavirus infections.
The group discovered that each coronavirus particle is unique, both in its underlying shape and its display of spikes. Few are spherical, bald, and bristle with spikes.
The spikes are floppy and move around, and we used a combination of tools to explore all their possible angles and orientations.”
Grigore Pintilie, Scientist, Department of Bioengineering, and Microbiology and Immunology, Stanford University
He has developed detailed 3D models of the virus and its spikes. He also adds each spike is different from all the rest, mainly in its direction and degree of tilting.
The scientist’s team reported their research in Nature Communications.
Since the pandemic started, most studies have looked at the structures of coronavirus spike proteins that were not attached to the virus itself. These are the first images made of the spikes of this strain of coronavirus while they’re still attached to the virus particles.”
Wah Chiu, Professor and Co-director, Department of Bioengineering, and Microbiology and Immunology, Stanford University
SARS-Cov-2’s More Benign Cousin
The research has origins during the initial days of the epidemic when research at SLAC shut down except for work aimed at understanding, preventing, and treating COVID-19 infections.
Due to the stringent safety requirements associated with experiments involving the actual SARS-CoV-2 virus, which necessitate high-level biosafety labs (BSL3), many researchers opt to study less harmful members of the coronavirus family.
In this particular investigation, Chiu and his colleagues focused on the human coronavirus NL63. This virus is responsible for up to 10% of human respiratory infections, primarily affecting children and immunocompromised individuals. The symptoms associated with human coronavirus NL63 span from mild coughs and sniffles to more severe conditions such as bronchitis and croup.
In 2020, Chiu and the team employed cryogenic electron microscopy (cryo-EM) along with computational analysis to capture images of NL63 spike crowns at near-atomic resolution.
However, due to the significantly thinner nature of the spike's stalk compared to its crown, they encountered challenges in obtaining clear, high-resolution images of both components simultaneously.
Zooming In on Spikes
This research combined information gleaned from a series of experiments to get a much more complete picture.
To begin, Stanford graduate student David Chmielewski employed cryogenic electron tomography (cryo-ET). This technique involves combining cryo-electron microscopy (cryo-EM) images of viruses, captured from various angles, to generate high-resolution 3D images of over a hundred NL63 particles.
SLAC senior scientist Michael Schmid plugged the images into a 3D visualization tool and found that each of a particle’s spikes is bent uniquely. Another SLAC scientist, Muyuan Chen, utilized advanced image reconstruction to create maps showing the average density of the spikes’ crowns and stalks.
In a detailed investigation, biological chemist Lance Wells from the University of Georgia utilized mass spectrometry to precisely identify the specific chemical compositions of the 39 sugar chains linked to each of the spike's three identical proteins.
Abhishek Singharoy, a computational biophysicist at Arizona State University, along with his student Eric Wilson, amalgamated these measurements to generate atomic models of the spikes' crowns and stalks at various bending angles. Additionally, they conducted simulations to explore the extent and flexibility of a spike's bending capacity.
It turns out that no matter what, the spikes have a preferred bending angle of about 50 degrees. They can tilt up to 80 degrees in any direction in the simulation, which matches well with our cryo-ET experimental observations.”
Wah Chiu, Professor and Co-Director, Department of Bioengineering, and Microbiology and Immunology, Stanford University
The flexion of the spike occurred in a specific region on the stalk, situated just below the crown, where a specific cluster of sugar molecules adhered to the protein, creating a hinge. Computer simulations indicated that alterations in the structure of this hinge could influence its flexibility.
Laboratory experiments further validated this hypothesis by demonstrating that mutations in the protein segment of the hinge significantly reduced the spike's infectivity. This implies that focusing on the hinge could potentially offer a viable avenue for combatting the virus.
“People working on the more dangerous coronaviruses, including MERS-CoV and SARS-CoV-2, have identified a region equivalent to this one and discovered antibodies targeting this region. That tells us it’s a critical region that is highly conserved, meaning that it has stayed much the same throughout evolution. So maybe by targeting this region in all coronaviruses, we can come up with a universal therapy or vaccine,” notes Jin.
Source:
Journal reference:
Chmielewski, D., et al. (2023). Structural insights into the modulation of coronavirus spike tilting and infectivity by hinge glycans. Nature Communications. doi.org/10.1038/s41467-023-42836-9