Singular protein infrared vibrational spectrum is seen by use of sophisticated measurement methods based on near-field optical microscopy.
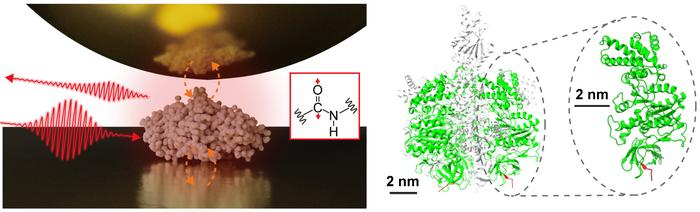
Single Protein Infrared Vibrational Spectroscopy (Left) Scheme Of Near-Field Infrared Spectroscopy Measuring A Single Protein. (Right) The Structure Of The Protein Complex F1-Atpase And The Subunit Measured In This Study. Image Credit: Jun Nishida
This technique makes use of light that is limited to the nanoscale, which makes it possible to analyze very small samples in detail - something that was previously difficult to do with traditional infrared spectroscopy. This accomplishment is a significant step toward the development of technical advancements like single-molecule vibrational spectroscopy and ultra-sensitive and super-resolution infrared imaging.

Image Credit: Konstantin Kolosov/Shutterstock.com
Because infrared spectroscopy can quantify vibrational spectra, often known as “molecular fingerprints,” it is frequently utilized for the structural and chemical investigation of a wide range of materials.
Ultra-high sensitivity and super-resolution infrared imaging are in greater demand due to the recent rapid growth of nanotechnology. However, detecting really small samples or obtaining spatial resolution at the nanoscale is beyond the capabilities of traditional infrared spectroscopy. It is hard to quantify a single protein, for instance, because even very sensitive infrared microspectroscopy needs over a million proteins to produce an infrared spectrum.
Using sophisticated measurement methods based on near-field optical microscopy, an interdisciplinary research team at the Institute for Molecular Science led by Jun Nishida, Assistant Professor, and Takashi Kumagai, Associate Professor, has successfully observed vibrational spectra of single proteins, which consist of approximately 500 amino acid residues. This technique makes use of light that is limited to the nanoscale, which makes it possible to analyze very small samples in detail - something that is difficult to do with traditional infrared spectroscopy.
The researchers used a gold substrate to isolate a single protein - a subunit of a protein complex known as F1-ATPase - and then conducted near-field infrared spectroscopy studies in a natural setting. The researchers made a significant advancement by successfully obtaining the infrared vibrational spectrum of a single protein, which could help characterize the local structural arrangements of specific proteins.
With improved insights into the mechanisms and interactions of membrane proteins and protein complexes, this information is especially crucial for understanding its complicated roles. Moreover, a novel theoretical framework explaining the nanoscale interactions between the protein and the infrared near field has been created.
The group was able to statistically replicate the experimental vibrational spectra they saw by using the theory. These findings will open the door to a variety of uses for nanoscale infrared spectroscopy, including the chemical study of biomolecules and other nanomaterials.
Source:
Journal reference:
Nishida, J., et al. (2024). Sub-Tip-Radius Near-Field Interactions in Nano-FTIR Vibrational Spectroscopy on Single Proteins. ACS Publications. doi.org/10.1021/acs.nanolett.3c03479