The RIKEN Center for Brain Science (CBS) employed genome mutation analysis to investigate the genetics of autism spectrum disorder (ASD) in people and their families. They found that the way a certain type of genetic mutation contributes to the illness differs from that of ordinary mutations.
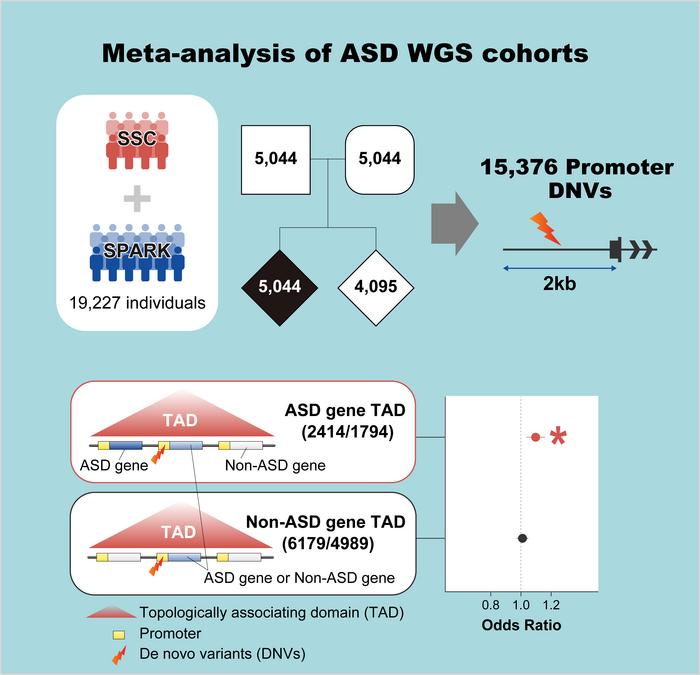 In this study, researchers analyzed large ASD whole genome sequencing data and found that promoter de novo mutations in tads containing ASD genes were specifically associated with the disease. Image Credit: RIKEN
In this study, researchers analyzed large ASD whole genome sequencing data and found that promoter de novo mutations in tads containing ASD genes were specifically associated with the disease. Image Credit: RIKEN
In summary, ASD can arise even in the absence of direct changes to ASD-related genes due to the three-dimensional structure of the genome, which allows mutations to impact nearby genes associated with ASD. On January 26th, this study was published in the scholarly journal Cell Genomics.
ASD is a collection of disorders that are partly defined by repetitive habits and trouble interacting with others. The genetics underlying its heritability are complicated and still largely unknown, despite the fact that it runs in families.
Research has demonstrated that examining the region of the genome responsible for encoding proteins alone is insufficient to account for the high level of heredity. The non-coding portions of the genome, especially promoters, which are responsible for determining whether or not proteins are really generated, may hold the key to the solution.
In these regions of the genome, "de novo" gene variants new mutations that are not inherited from one's parents were investigated by the RIKEN CBS team under the direction of Atsushi Takata.

Image Credit: Kateryna Kon/Shutterstock.com
This is one of the largest genome-wide studies of ASD conducted to date, with the researchers having examined a massive dataset spanning over 5,000 families. They concentrated on TADs, which are three-dimensional genomic structures that facilitate interactions between many neighboring genes and their regulatory components.
Researchers discovered that promoter de novo mutations increased the incidence of ASD only in cases where the promoters were identified in TADs containing genes linked to ASD. These de novo mutations have the potential to impact the expression of genes linked to ASD because they are in the same TAD and close by.
The current study provides an explanation for why mutations can raise the risk of ASD even in cases when they are not found in areas that code for proteins or in the promotors that directly regulate the expression of genes linked to ASD.
Our most important discovery was that de novo mutations in promoter regions of TADs containing known ASD genes are associated with ASD risk, and this is likely mediated through interactions in the three-dimensional structure of the genome.”
Atsushi Takata, Corresponding Author, Laboratory for Molecular Pathology of Psychiatric Disorders, RIKEN Center for Brain Science
Using the CRISPR/Cas9 technology, the researchers altered the stem cells' DNA to validate this by introducing mutations in particular promoters. They saw, as predicted, that within the same TAD, a single genetic modification in a promotor led to changes in an ASD-associated gene.
Takata compares the procedure to a genomic "butterfly effect," in which a single mutation dysregulates disease-associated genes that are dispersed in far parts of the genome. This is because several genes linked to ASD and neurodevelopment were also damaged in the mutant stem cells.
According to Takata, this discovery has consequences for the creation of fresh approaches to diagnosis and treatment.
At the very least, when assessing an individual’s risk for ASD, we now know that we need to look beyond ASD-related genes when doing genetic risk assessment, and focus on whole TADs that contain ASD-related genes.”
Atsushi Takata, Study Corresponding Author, Laboratory for Molecular Pathology of Psychiatric Disorders, RIKEN Center for Brain Science
Takata explained, “Further, an intervention that corrects aberrant promoter-enhancer interactions caused by a promotor mutation may also have therapeutic effects on ASD.”
To gain a deeper knowledge of the genetic foundations of ASD, further research involving more families and patients is needed.
By expanding our research, we will gain a better understanding of the genetic architecture and biology of ASD, leading to clinical management that enhances the well-being of affected individuals, their families, and society.”
Atsushi Takata, Corresponding Author, Laboratory for Molecular Pathology of Psychiatric Disorders, RIKEN Center for Brain Science
Source:
Journal reference:
Nakamura, T., et.al., (2024). Topologically associating domains define the impact of de novo promoter variants on autism spectrum disorder risk. Cell Genomics. doi.org/10.1016/j.xgen.2024.100488