By using a novel neuronal cell culture model, researchers at the University of Zurich have illuminated the complex mechanisms behind neurodegeneration. Their study identified a malfunctioning protein as a potential target for therapy in the management of FrontoTemporal Dementia (FTD) and Amyotrophic Lateral Sclerosis (ALS).
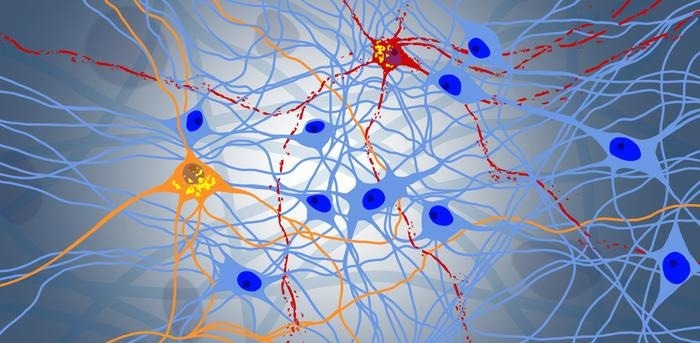 Progressive degeneration in a neuronal network: blue represents healthy neurons, while orange and red represent the protein NPTX2. Yellow shows the toxic aggregation of the protein TDP-43. Image Credit: Niklas Bargenda
Progressive degeneration in a neuronal network: blue represents healthy neurons, while orange and red represent the protein NPTX2. Yellow shows the toxic aggregation of the protein TDP-43. Image Credit: Niklas Bargenda
Depending on the area of the brain that is affected, neurodegenerative illnesses cause some of the neurons in the brain to die, producing a variety of symptoms. Paralysis results from the degeneration of neurons in the motor cortex and spinal cord in Amyotrophic Lateral Sclerosis (ALS).
On the other hand, neurons in the areas of the brain related to cognition, language, and personality are impacted in FrontaTemporal Dementia (FTD).
Both ALS and FTD are incurably progressing illnesses for which there are now no reliable treatments. Age-related neurodegenerative disorders like ALS and FTD are predicted to become more common as the population ages.
The majority of ALS and around half of FTD patients have abnormal buildup of a protein called TDP-43 in their central nervous system neurons; nevertheless, the underlying molecular pathways causing neurodegeneration are still mostly understood.
Flexible, Durable, Reproducible: Ideal Cell Culture Model for ALS and FTD Research
Marian Hruska-Plochan, the study's first author, and Magdalini Polymenidou, the study's corresponding author, from the University of Zurich's Department of Quantitative Biomedicine created a unique brain cell culture model that mimics TDP-43's abnormal behavior in neurons.
They found a hazardous increase in the protein NPTX2 using this model, indicating that it may be a viable therapeutic target for FTD and ALS.
Marian Hruska-Plochan created a novel cell culture model dubbed "iNets" using human induced pluripotent stem cells to simulate neurodegeneration. These cells can be used to create a wide variety of desirable cell types since they are derived from skin cells that were reprogrammed to an extremely early, undifferentiated stage in the lab.
An interconnected network of neurons and the cells that support them that grow in layers is called an iNet.
The cultures were simple to replicate and had an incredibly long shelf life of up to a year.
The robustness of aging iNets allows us to perform experiments that would not have been possible otherwise, and the flexibility of the model makes it suitable for a wide range of experimental methodologies.”
Marian Hruska-Plochan, Study First Author, Department of Quantitative Biomedicine, University of Zurich
As an illustration, the iNets cell cultures offered the perfect model to study the development of neurodegeneration from TDP-43 malfunction.
How Protein Dysfunction Leads to Neurodegeneration
By using the iNets model, the researchers were able to determine that the missing link between TDP-43 misbehavior and neuronal death was a toxic accumulation of NPTX2, a protein that is normally produced by neurons through synapses.
They studied the brain tissue of ALS and FTD patients who had passed away to verify their theory, and they discovered that NPTX2 did accumulate in the patient's brains when aberrant TDP-43 was present. This indicates that the pathophysiology of ALS and FTD patients was correctly anticipated by the iNets culture model.
The researchers investigated whether NPTX2 could be a target for drug design to treat ALS and FTD in further studies conducted in the iNets model. The group devised a plan whereby they reduced NPTX2 levels in neurons exhibiting TDP-43 misbehavior.
They discovered that preventing neurodegeneration in the iNets neurons involved lowering NPTX2 levels. Thus, medications that lower the level of the protein NPTX2 may be used as a therapeutic approach to stop neurodegeneration in people with FTD and ALS.
We still have a long way to go before we can bring this to the patients, but the discovery of NPTX2 gives us a clear shot of developing a therapeutic that acts at the core of the disease, and in conjunction with two additional targets recently identified by other research teams, it is conceivable that anti-NPTX2 agents could emerge as a key component of combination therapies for ALS and FTD in the future.”
Marian Hruska-Plochan, Study First Author, Department of Quantitative Biomedicine, University of Zurich
Source:
Journal reference:
Hruska-Plochan, M., et al. (2024) A model of human neural networks reveals NPTX2 pathology in ALS and FTLD. Nature. doi.org/10.1038/s41586-024-07042-7