Scientists from the University of Bath and UCB—a biopharma company—have identified a new method to synthesize miniaturized antibodies, paving the way for a promising new class of therapies for various diseases.
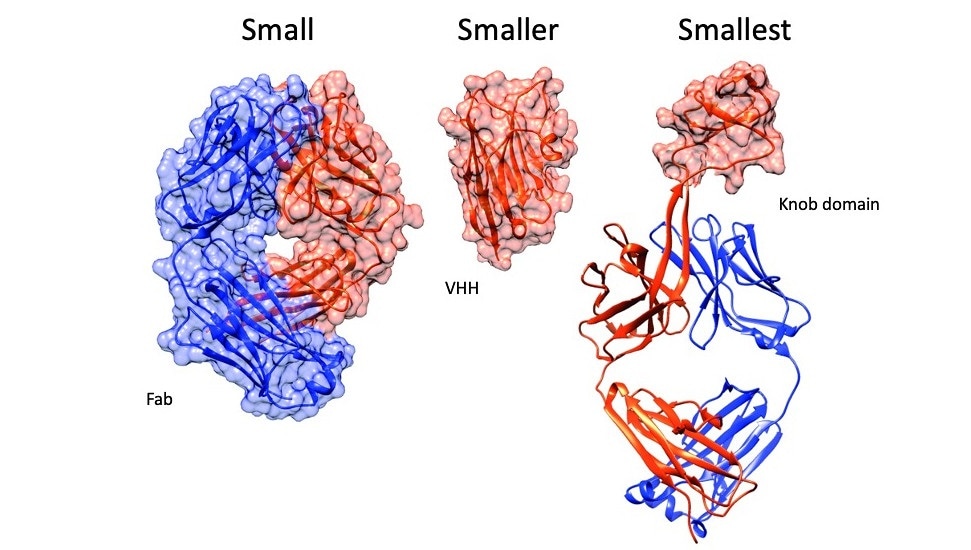
From left to right: The antigen binding fragment of an antibody (Fab), an antibody fragment consisting of a single domain antibody (VHH) and a knob domain. Image Credit: University of Bath.
To date, the smallest manmade antibodies, called monoclonal antibodies or mAbs, were extracted from sharks, llamas, and alpacas; however, the breakthrough molecules that were extracted from the immune cells of cows were found to be five times smaller. This can be attributed to an unusual characteristic of a bovine antibody known as a knob domain.
The diminutive size of the novel antibodies holds huge potential medical implications. For example, the antibodies may attach to sites on pathogens that otherwise are too large for normal antibody molecules to attach to, causing the destruction of invasive microorganisms. The new antibodies may also be able to gain access to the body’s sites which cannot be done by larger antibodies.
Antibodies contain chains of amino acids—the building blocks of proteins—that combine together to form a loop-like structure.
The loops within the chains, called complementarity identifying regions, attach to antigen targets, thus triggering the immune system. Among all the antibodies, bovine antibodies are loopier and about 10% include a knob domain—a unique feature among jawed vertebrates. These closely packed bundles of mini-loops are expressed on a protein stalk, far from other loops, and are believed to play a crucial role in binding.
There is a simple reason as to why the knob domains are creating a stir: separated from the rest of the antibody, such loop extensions can work independently, effectively rendering small antibodies that can attach tightly to their targets.
These knobs are able to bind their target as complete antibodies, so in effect we have been able to miniaturise antibodies for the first time.”
Jean van den Elsen, Professor, Department of Biology and Biochemistry, University of Bath
Elsen, who was involved in the study, stated that the outcome was rather surprising.
These novel molecules have been designed as part of a joint project between the University of Bath and UCB, a global biopharma company. The molecules originate from cows that have been immunized by an antigen injection, triggering an immune response. Incidentally, antigens are particles of a foreign body.
Natural antibodies are extracted from the cow via a process of sorting and “deep sequencing” of antibody synthesizing B-cells. The antibodies, thus obtained, are then produced in the laboratory in cultures of human cells.
Normal antibodies are produced by the human body as part of its natural reaction to an infection, while monoclonal antibodies are injected into a patient when an infection has already taken hold and they are finding it hard to beat it unaided.
In the last few decades, mAbs have evolved as effective therapies for different medical disorders, such as autoimmune disorders, cancers, and serious viral infections. It is believed that miniaturized mAbs will ultimately be involved in a series of drug treatments.
The antigen, employed by the researchers from the University of Bath to trigger an immune response in cows, is known as Complement component C5. This antigen plays a vital role in several human diseases, like COVID-19, where there is an inflammatory response.
In addition to having an advantage over normal mAbs, the novel monoclonal antibodies are also stronger, which means they remain stable for a longer period of time.
Professor van den Elsen added, “They have very sturdy, tightly packed structures. So not only do they get to places better than other antibodies but they may also have a far longer shelf life.”
Antibody drug discovery is an established field but this research opens up entirely new opportunities. There is huge potential use for these miniaturised antibodies.”
Alex Macpherson, Study Lead Author and PhD Student, University of Bath
Macpherson is also a biochemist at UCB.
Alastair Lawson, immunology Fellow at UCB and also the UCB lead on the project, stated, “This research has led to the discovery of the smallest clinically relevant antibody fragments ever reported and we are very excited about their potential.”
Source:
Journal reference:
Macpherson, A., et al. Isolation of antigen-specific, disulphide-rich knob domain peptides from bovine antibodies. PLOS Biology. doi.org/10.1371/journal.pbio.3000821.