Researchers have achieved, for the first time, a look into the complex workings of a cell from within the cell. By introducing minuscule tracking devices (that look like tiny spiders) directly into the interior of egg cells when they fertilize, they have found the processes that regulate the beginning of development.
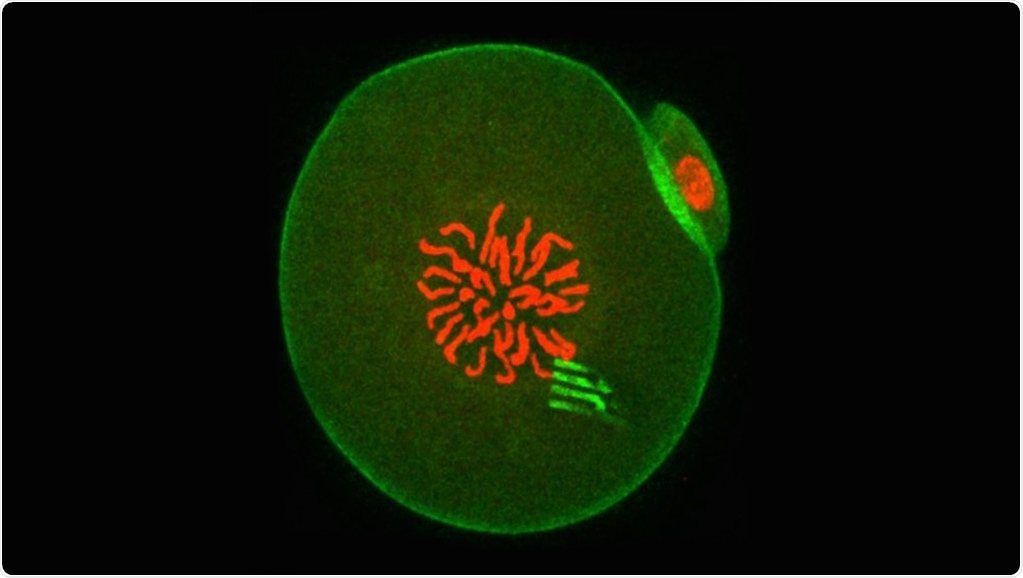
Fluorescence of an embryo containing a nanodevice. Image Credit: University of Bath.
This study on one-cell embryos could reform human understanding of the mechanisms that usually underlie cellular behavior, and may eventually offer insights into what goes wrong in disease and aging.
Led by Professor Tony Perry from the Department of Biology and Biochemistry at the University of Bath, the team injected a silicon-based nanodevice along with sperm into the egg cell of a mouse. The outcome was a healthy, single-cell embryo including a tracking device.
The small devices act somewhat like spiders, with eight extremely flexible “legs.” The legs quantify the “pushing and pulling” forces applied within the interior of the cell at a higher level of precision, thus unraveling the cellular forces at play and revealing how intracellular matter reorganized itself in time.
The nanodevices are extremely thin—similar to structural components of some cells, and measuring 22 nm, which makes them nearly 100,000 times thinner than a pound coin. This implies they have the versatility to record the movement of the cell’s cytoplasm as the single-cell embryo starts its journey of becoming a two-cell embryo.
This is the first glimpse of the physics of any cell on this scale from within. It’s the first time anyone has seen from the inside how cell material moves around and organises itself.”
Tony Perry, Professor, Department of Biology and Biochemistry, University of Bath
Why probe a cell’s mechanical behavior?
According to Professor Perry, the activity inside a cell governs the functions of that cell.
“The behavior of intracellular matter is probably as influential to cell behaviour as gene expression,” he added.
But this complex play of cellular material has mostly been unexplored until now as it is technically difficult. Thus, the team has been able to find the elements that constitute a cell, but not the behavior of the cell interior as a whole.
From studies in biology and embryology, we know about certain molecules and cellular phenomena, and we have woven this information into a reductionist narrative of how things work, but now this narrative is changing.”
Tony Perry, Professor, Department of Biology and Biochemistry, University of Bath
The narrative was authored principally by biologists, who brought with them the tools and questions of biology. What was lacking was physics. Physics questions about the forces that drive the behavior of a cell, and offers a top-down method to find the solution.
Professor Perry stated, “We can now look at the cell as a whole, not just the nuts and bolts that make it.”
Mouse embryos were selected for the study due to their comparatively large size (they measure 100 µm, or hundred-millionths of a meter, in diameter, compared to a standard cell that has a diameter of only 10 µm [ten-millionths of a meter]). This signifies that each embryo includes a space to hold a tracking device.
The team performed the measurements by studying video recordings captured under a microscope as the single-cell embryo developed.
Sometimes the devices were pitched and twisted by forces that were even greater than those inside muscle cells. At other times, the devices moved very little, showing the cell interior had become calm. There was nothing random about these processes - from the moment you have a one-cell embryo, everything is done in a predictable way. The physics is programmed.”
Tony Perry, Professor, Department of Biology and Biochemistry, University of Bath
The study findings add to the latest picture of biology that reports material within a living cell is not stationary, but alters its properties in a pre-ordained way as the cell carries out its role or responds to the environment. The study could someday have implications for human interpretation of how cells age or stop working as they should, which is what occurs in disease.
Source:
Journal reference:
Duch, M., et al. (2020) Tracking intracellular forces and mechanical property changes in mouse one-cell embryo development. Nature Materials. doi.org/10.1038/s41563-020-0685-9.