Globally, chronic obstructive pulmonary disease, or COPD, is the third most common cause of mortality. It is characterized by permanent and irreversible lung damage, for which lung transplantation is the only effective treatment. Unfortunately, it is hard to find good lung donors.
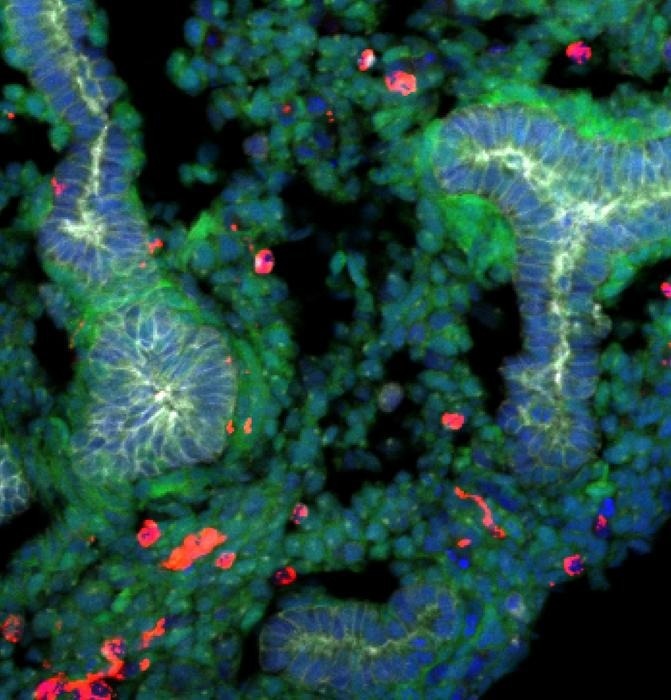 A rat embryonic stem cells derived lung generated within a mouse body stained with epithelial cells (labeled with E-cadherin, shown in gray), rat cells (shown in green), mouse cells (shown in red), and DNA (labeled with Hoechst, shown in blue). Rat cells are predominantly detected in the lung, and the epithelial tissues are exclusively composed of rat cells. Image Credit: Shunsuke Yuri
A rat embryonic stem cells derived lung generated within a mouse body stained with epithelial cells (labeled with E-cadherin, shown in gray), rat cells (shown in green), mouse cells (shown in red), and DNA (labeled with Hoechst, shown in blue). Rat cells are predominantly detected in the lung, and the epithelial tissues are exclusively composed of rat cells. Image Credit: Shunsuke Yuri
Regenerative medicine is making progress in creating lungs from pluripotent stem cells (PSCs) using interspecies animal models to make up for this lack of donors. Interspecies chimeric animals can be produced by injecting PSCs and embryonic stem cells (ESCs) from one species into the blastocysts of another species that lack an organ, a biological process known as blastocyst complementation.
In rat-mouse chimeras, this approach has successfully allowed for the regeneration of the kidney, heart, and pancreas. Nevertheless, successful functional lung creation remains elusive, indicating the need for additional investigation into the feasible circumstances necessary to produce organs produced from pluripotent stem cells.
Reverse-blastocyst complementation (rBC) has now been employed by scientists at the Nara Institute of Science and Technology (NAIST) in Japan to better understand the circumstances necessary for lung formation in rat-mouse chimeric models. In addition, scientists successfully created a rat-derived lung in their mouse model using the tetraploid-based organ complementation (TOC) approach.
Leading the research were Ayako Isotani and Shunsuke Yuri from NAIST, and the study was published in Development. Lung development depends on the fibroblast growth factor 10 (Fgf10) and how it interacts with the Fgf receptor 2 isoform IIIb (Fgfr2b) in the lungs. In this study, mutant ESCs that do not exhibit lung development were injected into wild-type (WT) embryos using the rBC technique.
This technique makes it possible to identify mutant PSCs in the recipient tissue with efficiency, which helps identify the prerequisites for lung development in the organ-deficient animal. Additionally, the research team discovered that in the chimeras, WT ESCs contribute consistently to both target and non-target organs.
This made it possible to determine that, in Fgf10- or Fgfr2b-deficient animals, a specific quantity of WT or normal cells is needed to overcome lung development failure.
With this information, they were able to apply the TOC approach to grow rat-derived lungs successfully in Fgfr2b-deficient mouse embryos without the requirement to create a mutant mouse line.
Interestingly, we found that the rat epithelial cells conserved intrinsic species-specific timing in the interspecies model, resulting in an underdeveloped lung.”
Shunsuke Yuri, Associate Professor, Nara Institute of Science and Technology
As a result, these lungs continued to be nonfunctional after birth. The results of this study unequivocally point out the conditions that must be met and the obstacles that must be removed in order for rat-mouse interspecies chimeras to generate functional lungs successfully, regarding the importance of these discoveries.
Yuri concludes, “We believe that our study makes an important contribution to the literature by presenting a faster and more efficient method of exploring blastocyst complementation. These novel results can significantly advance the progress toward developing in-vivo chimeric lungs for the purpose of transplantation, which could transform the practical application of regenerative medicine.”
Source:
Journal reference:
Yuri, S., et.al., (2024). Generation of rat-derived lung epithelial cells in Fgfr2b-deficient mice retains species-specific development. Development. doi.org/10.1242/dev.202081.