This article is based on a poster originally authored by Jonathan Sheard, Essi M. Niemi and Piia Mikkonen1 and was presented at ELRIG Drug Discovery 2023.
1UPM Biomedicals

Introduction
GrowDex®, GrowDex®-T, and GrowDex®-A are birch-based nanofibrillar cellulose (NFC) hydrogels used for 3D cell culture and, aside from NFC, only consist of purified water. GrowDex hydrogels include no animal or human-derived materials.
GrowDex hydrogels, which resemble the extracellular matrix (ECM), promote 3D cell development and are biocompatible with human cells and tissues. The hydrogels’ structure and mechanical characteristics can be tailored to meet the needs of various cell types (Figure 1), and they promote the free flow of nutrients and oxygen. Cellulase enzyme may break down GrowDex to soluble glucose while maintaining the 3D structure of the cells.
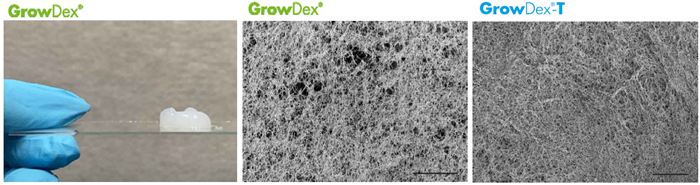
Figure 1. Macroscopic image of native hydrogel and SEM images of native and anionic hydrogels (bars 5 μm). SEM Images by Donata Iandolo from the University of Cambridge, UK.
GrowDex hydrogels can be tuned for a variety of applications, such as automated 3D cell-based high-throughput (HTS) and high content screening (HCS) assays for drug development. GrowDex hydrogels also have excellent shear thinning properties and temperature stability.
Key Properties
Modeling the Tumor Immune Environment
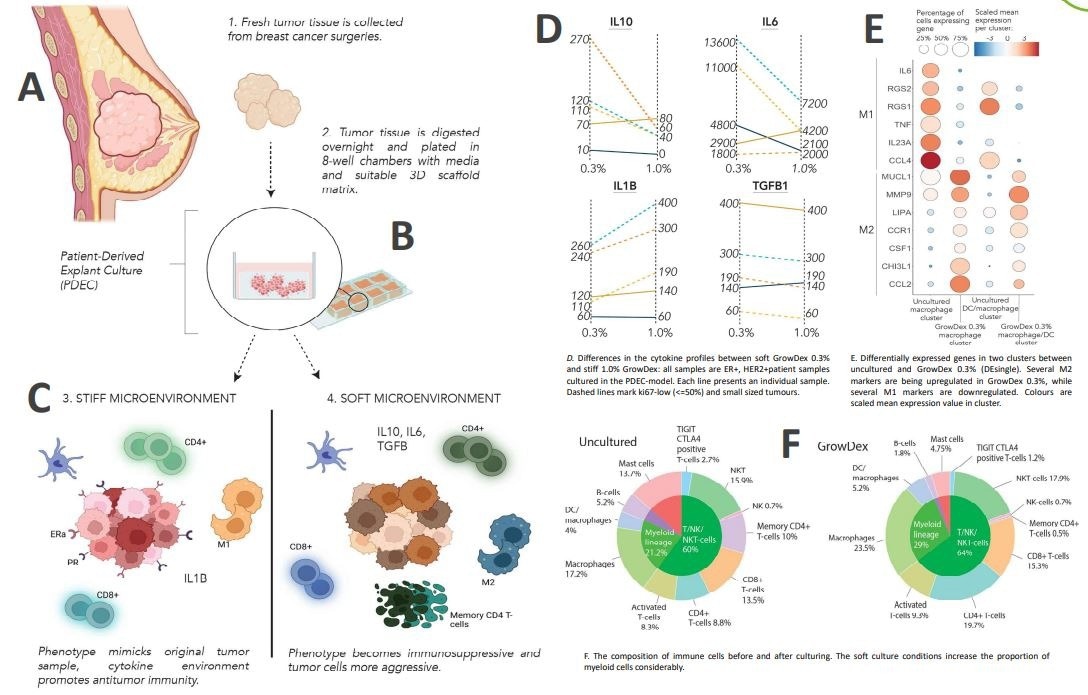
Fresh patient-derived tumor samples were used for patient-derived explant culture (PDEC) using GrowDex (previously reported in Haikala et al. 2019 and Munne et al. 2021).
To be more precise, after breast cancer surgery (A), new tumor tissue was effectively obtained, separated, and implanted in two distinct GrowDex hydrogel concentrations: 0.3% and 1% (B). After the cells were cultured for three to seven days, the cytokine release profile (D), bulk sequencing (E), and single-cell analysis (F) were examined.
GrowDex hydrogels have demonstrated the ability to maintain the tumor microenvironment, together with the implanted immune cells, for five to seven days.
It was shown that by modifying the mechanical and biological characteristics of the matrix, it was possible to concurrently change the tumor immune microenvironment and phenotype.
The tumor phenotype resembled the original tumor in a stiff microenvironment (1% GrowDex), and the cytokine environment enhanced anti-tumor immunity.
On the other hand, the tumor cells grew more aggressive and phenotypic immunosuppressive in a soft microenvironment with 0.3% GrowDex.
Simulating the stiffness and other biological characteristics of the tumor microenvironment is feasible using PDECs in conjunction with GrowDex hydrogel. These characteristics subsequently influence the tumor phenotype and antitumor immunity.
The cytokine environment is changed in a soft microenvironment (0.3% GrowDex), which also causes memory CD4+ T-cell depletion and changes macrophage polarization to an M2 immunosuppressive phenotype. Researchers are currently trying to figure out the precise mechanism that causes this phenomenon.
Conclusions
- Animal-free GrowDex hydrogels are biocompatible with cells and tissues, making them appropriate for primary tumor cultivation and tumor-associated immune cells.
- They are especially well suited for sensitive drug discovery investigations with reproducible workflows since their composition is well determined and there is no batch variation.
- The study emphasizes how crucial it is to have reproducible, precise in vitro cell culture models to accurately model diseases and do drug screening.
- Biologically Relevant GrowDex hydrogels are an excellent choice as their characteristics give the cells a 3D microenvironment ideal for the target phenotype.
Acknowledgment and Reference
Pauliina Munne and Aino Peura from the Klefström Lab. Aino Peura et. al. Immune Evasion in Soft Nanocellulose 3D Matrix, Poster presented at Cold Spring Harbor 2022. Haikala, et.al.2019, and Munne et. al. 2021
Last Updated: Jul 8, 2024