Plasmapheresis and the history of bloodletting
Bloodletting was a highly popular historic therapy with some applications in the modern-day. Arising from the concept of "humors," Hippocrates (~460-370 BC) believed that humans were composed of four basic elements or humors: blood, phlegm, black bile, and yellow bile. Each humor was associated with an organ (brain, lungs, spleen, and gallbladder, respectively) and a personality type (sanguine, phlegmatic, melancholic, and choleric). An imbalance of the four humors was thought to be the cause of an illness, and removing the abundant humor would restore the balance.
One such removal method was bloodletting, common in the 1st century but further popularized by Galen (129-200 AD), who claimed that blood was the "dominant" humor. Bloodletting became a regular practice through his teachings, especially in the Middle Ages. The concept was fundamentally flawed and lost popularity by the 19th century when other scientific methods showed greater success. Though humors have long been discarded, ironically, blood itself has four major components (red blood cells, white blood cells, platelets, and plasma), which can be separated and removed as a potential therapy for certain diseases.
Plasmapheresis, a common treatment in Europe and Japan, is an extracorporeal procedure where the plasma component is separated and removed from the blood of a patient. It is usually followed by plasma exchange, wherein the removed plasma is replaced with a donor's. This procedure is common and generally used to mediate the levels of circulating plasma proteins and, in theory, removes an imbalance of circulating plasma proteins to alleviate the symptoms of specific diseases.
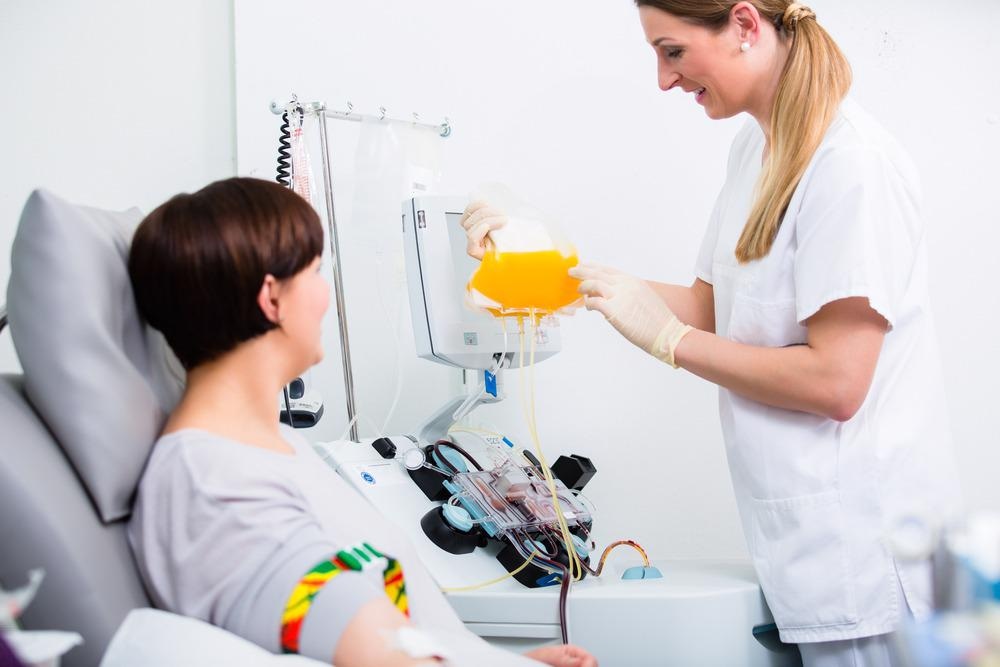
Image Credit: Kzenon/Shutterstock.com
Understanding the plasmapheresis procedure
The procedure itself falls under the term "apheresis," which generally describes procedures that extract blood, remove a component, and return the remainder of the blood to the host. Plasmapheresis consists of two main techniques: centrifugation and filtration. In any apheresis procedure, anticoagulants must be added to prevent extracorporeal clotting, and replacement fluid is added, depending on the condition being treated. Generally, plasmapheresis is considered safe and well-tolerated, with considerations varying depending on the treated condition and the replacement fluid solution.
Centrifugation apheresis spins extracted blood at high speeds to separate it into the four major components by density; this is commonly done at blood banks where plasma can be collected and donated. Filtration plasmapheresis passes blood through a filter to separate plasma from larger cells and platelets in the blood, then returns the fluid (sans plasma) to the patient alongside a predetermined replacement fluid. This is commonly used by nephrologists and performed via dialysis machines. It's a more efficient method of separating and removing plasma. However, it requires specialized filters, which can be problematic if some plasma components exceed the size of available filters.
Clinical interventions using plasmapheresis
There are many proposed therapeutic applications for plasmapheresis, which are separated into categories. Category 1 includes disorders that can be treated with plasmapheresis as a first-line therapy, category 2 are diseases using plasmapheresis as a second-line treatment, and category 3 disorders are those in which the benefit of plasmapheresis is minimal. Category 4 involves disorders where plasmapheresis is ineffective or harmful.
Myasthenia gravis (MG) is a chronic autoimmune condition that causes muscle weakness without atrophy because of a defect in the action of acetylcholine at the neuromuscular junction. MG is a category 1 disorder, but plasmapheresis is a short-term crisis intervention as with many similar chronic autoimmune conditions. A standard course for patients with MG consists of five exchanges on alternating days utilizing 2-4 liters per exchange; the effect of the plasmapheresis generally lasts up to 6 weeks meaning that regular immunosuppressive or immunomodulatory agents need to be co-administered.
Alternatively, a case study that used plasmapheresis to treat a patient with a history of intrauterine fetal death due to severe fetal anemia showed that it might delay the development of fetal anemia. The patient's condition was red cell alloimmunization in pregnancy which causes hemolytic disease in the fetus and newborn, resulting in fetal anemia, fetal hydrops, and fetal death. The standard is the antenatal treatment of intrauterine transfusion but can only be feasible after 20 weeks of gestation. On the other hand, plasmapheresis is useful for cases that occur at less than 20 weeks gestation or in cases where intrauterine transfusion is unfeasible, such as in this case study where the patient had an unsuitable umbilical cord position for transfusion.
The patient underwent selective plasma exchange (SePE) once a week from week 17 onwards, performed twice a week when antibody levels were raised at around 20 weeks. The purpose was to reduce immunoglobulin G (IgG) levels from the maternal circulation, reducing the amount of alloantibody transported to the fetus. The fetus was eventually born by cesarean section and needed phototherapy for the first 11 days, and five transfusions of 10 ml/kg red blood cells and eventually discharged at day 46.
Plasmapheresis is a specialized treatment option for a variety of diseases
A variety of immune or general plasma-based disorders can receive short-term symptomatic relief using plasmapheresis. As well as this, it can also be used as an adjunct to prepare for procedures, surgery, and postoperative recovery, and it is an intensive care procedure that requires specialized care and training with close monitoring and follow-up.
Sources:
- Nguyen, TC., et al. (2012) The Role of Plasmapheresis in Critical Illness. Crit Care Clin. doi.org/10.1016/j.ccc.2012.04.009
- Greenstone, G. (2010) The history of bloodletting. [Online] BCMJ. Available at: https://bcmj.org/premise/history-bloodletting. (Accessed on 1 February 2022).
- Drew, MJ. (2008) Plasmapheresis in the Dysproteinemias. Ther Apher. doi.org/10.1046/j.1526-0968.2002.00393.x
- Lehmann, HC., et al. (2006) Plasma Exchange in Neuroimmunological Disorders: Part 1: Rationale and Treatment of Inflammatory Central Nervous System Disorders. Arch Neurol. doi.org/10.1001/archneur.63.7.930
- Daga Ruiz, D., et al. (2017) Plasmapheresis and other extracorporeal filtration techniques in critical patients. Med Intensiva. doi.org/10.1016/j.medin.2016.10.005
- Basu, D. and Kulkarni, R. (2014) Overview of blood components and their preparation. Indian J Anaesth. https://doi.org/10.4103/0019-5049.144647
- Sieb, JP. (2014) Myasthenia gravis: an update for the clinician. Clin Exp Immunol. doi.org/10.1111/cei.12217
- Maki, Y., et al. (2020) Plasmapheresis for the Treatment of Anti-M Alloimmunization in Pregnancy. Case Rep Obstet Gynecol. doi.org/10.1155/2020/9283438
- Sergent, SR. and Ashurst, JV. (2021) Plasmapheresis. [Online] Statpearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK560566/. (Accessed on 1 February 2022).
Further Reading
Last Updated: May 16, 2022