Colorimetry has been around since the 1870s when Jules Dubosq invented the colorimeter. In 1885 Joseph William Lovibond devised a method of measuring colors with a handheld comparator while working in his father's brewery. He compared solutions (beer) to standard colors by viewing them side by side and using filters colored to the required shade.

Image Credit: smereka/Shutterstock.com
Colorimetry is a technique for measuring the concentration of a known constituent in a solution compared to standard solutions. The human eye perceives light and color in a very subjective way. Science demands an objective way of measuring things, which is colorimetry. Mathematically, the Beer-Lambert Law, which is a combination of two laws, defines colorimetry.
Beers Law says that if monochromatic light passes through a homogenous solution, the amount of light transmitted is directly proportional to the solute in the solution.
Lamberts Law states that the length of the path determines the absorbance of light by a colored solution and the volume of liquid it passes through
Beer Lambert's law states that A = Ecl
Where;
- A is absorbtivity
- C is concentration
- l is the length of the light path.
Colorimetry uses this law to measure the absorbance (more frequently) or light transmittance to determine solution concentrations. The solution must not fluoresce, and there should be no temperature change during the measuring process.
The perception of color by the human eye depends on the nature of the illumination, the optical properties of the object viewed, and the human eye's response. The human eye can detect light with a wavelength between 400nm and 700 nm, and it sees color with three types of cone cells. Blue cones are for short wavelengths, green for medium, and red for long wavelengths, leading to the term "tristimulus colors," which blend the three. Tristimulus color standards are laid down by the ISO (International Standards Organisation) standards or by the French CIE (Commission Internationale Eclairage, which translates as the International Commission on Illumination).
Color Comparators and Colorimeters
The principle of Lovibond's comparator is still in use today, and indeed the "Lovibond Comparator" is still a brand of the Tintometer company in the UK. This type of colorimeter, sometimes called a "Filter Photometer," consists of a handheld cube with space for two cuvettes into which liquids for analysis are placed. The filter is a disc with plastic or glass "filters" with progressively different intensities of color, which allows the comparison of the solution in question with a standard and the concentration measured. A standard light source added to a comparator can give more accurate results. This type of low-cost robust colorimeter is excellent for field analysis of water, foods, etc.
A comparator still relies on the human eye to interpret colors, which is subjective. It is desirable to make the process more objective and repeatable for scientific reasons. Tintometer, Hach, La Motte, Xylem, Hanna, and other companies have developed electronic instruments to eliminate human subjectivity. They make colorimeters and photometers for lab and fieldwork.
A colorimeter will typically consist of the following:
- An illuminant – a specific light source (daylight in a comparator), usually an LED or incandescent light, which projects a fixed, consistent light onto an object.
- An Observer – a specified field of view to analyze colors. A standard observer is usually two degrees in a colorimeter
- A Tristimulus absorption filter- to isolate specific wavelengths for the sample.
- Photoelectric cell – electronic colorimeters use a photoelectric cell to measure light and convert it into a digital readout
The advantages of this type of instrument are that they are focused on specific tristimulus values, so only one light source is required, which lowers the cost. They also give rapid results and are easily portable and suitable for in-field use. However, they are not very versatile and are focused on a small light spectrum, and the accuracy of results is sometimes not good enough for research and development. They cannot identify a metamerism (identical colors in one light but different in another). This instrument is used widely in field applications such as water treatment and inspection in manufacturing.
A photometer is slightly more sophisticated than a colorimeter. A Photometer's construction is similar but more versatile than a colorimeter.
- An illuminant – with a versatile, not fixed light source
- An Observer – with a wider field of view, typically around ten degrees.
- A Prism and– the prism separates the wavelengths of light, and t
- Grating interference filter - chooses which to analyze.
- Photoelectric cell – photometers a photoelectric cell to measure light and convert it into a digital readout.
A photometer is a more complex and versatile instrument that includes moving parts and is usually bench-mounted, so only suitable for lab work. These instruments are often used with complicated software and give highly accurate results.
A critical difference between colorimeters/comparators and photometers is what they measure. Colorimeters measure absorbance, whereas photometers measure transmittance or reflectance. Colorimeters typically have LED light sources, and photometers have tungsten, Xenon, or Deuterium light sources.
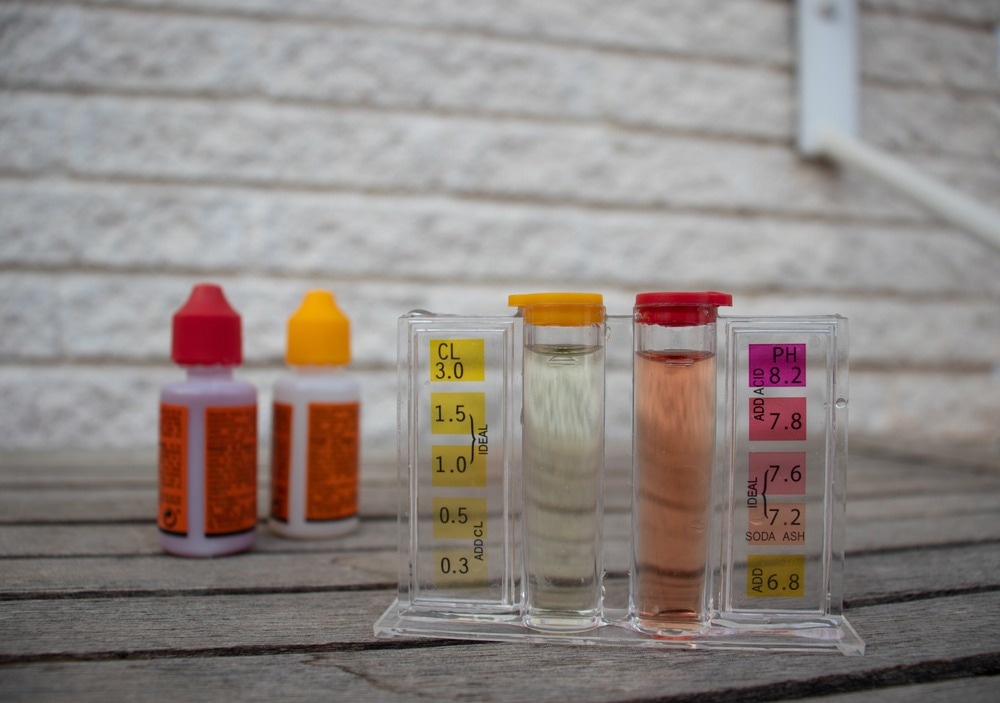
Image Credit: Zen HP/Shutterstock.com
Uses of Colorimetry
Colorimetry is used in monitoring to measure bacterial growth rates, quality, and color in food and beverage manufacturing, textiles manufacturing, cosmetics, paint works, and the petroleum industry. It is used in the pulp and paper industry to manage pulp's color and control printing colors. Colorimetry is an important tool in cosmetology to measure the color of skin and teeth. The quality of diamonds and other stones in gemmology is determined using Colorimetry.
In water treatment, Colorimetry finds many uses in consumer and industrial applications. It is used in swimming pools and hot tub management to maintain safe conditions. Industrial water treatment, utility water treatment, and wastewater analysis use Colorimetry to identify chemical and other contaminants.
In medicine, hemoglobin levels in the blood can be determined using Colorimetry. It is also used to measure cholesterol, oxygen levels, and enzymatic reactions.
Two or more reagents reacted together can produce a color for analysis by Colorimetry in a simple chemical reaction that creates a measured color. Sometimes the sample needs secondary treatment, such as enzyme treatment or ultraviolet light irradiation, to make the color that is measured. Using these more sophisticated treatments increases the number of parameters available for testing. The reagents or indicators are often powdered and packaged in tiny containers for easy transportation and storage, ideal for field testing. These techniques mean that fairly accurate testing for specified parameters can be carried out almost anywhere with a colorimeter. Researchers in laboratories can achieve highly accurate measurements using photometers.
Further Reading
Last Updated: Jan 12, 2023