Organic Chemistry is a field that requires a good amount of spatial reasoning, and a rooted knowledge of chemical structure. To accomplish organic chemistry with apropos results, one must follow the standards and nomenclature that the International Union of Pure and Applied Chemistry (IUPAC) provides, as well as the rules brought forth by valence shell electron pair repulsion (VSEPR).

Image Credit: Victor Moussa/Shutterstock.com
The first misconception of organic chemistry is that the public equates this field to physics or math, when in reality it lacks the veracity of the former, though it is still empirically driven. The qualitative results and guidelines that are bound to chemistry came from experimentations in the lab, while verification is left to spectroscopy, as well as other analytical means.
Fields like math may be more concrete in their modus operandi, while the study of carbon-contain life is intrinsically greyer and more nebulous, attributing to students’ difficulty to grasp it.
Electrophilic Aromatic Substitution
Misinterpretation and general confusion may come with substitutions reactions, which rely on many different variables. Though the energy gap between the highest occupied molecular orbital (HOMO), and the lowest occupied molecular orbital (LUMO) is reduced, apparent via energy diagrams, the resulting structure of the molecule is not. The product, the reactant, the general medium (acidic or basic), and often the catalyst, all affect the product obtained, as well as the general yield.
Take a benzene ring as an example. The pi electrons within the aromatic ring are of lower energy than simple alkenes, which requires a stronger (acidic) electrophile to overcome the aromatic stabilization. Therefore, not all electrophiles can perform these substitutions, a mistake that is easily made if one is not familiar with the electronegativity of a certain substituent. Some valid electrophiles for a typical electrophilic substitution performed on an aromatic reagent are halogens, nitronium ions, sulfonium ions, and acylium ions, which undergo halogenation, nitration, sulfonylation, and the Friedel-Crafts acylation respectfully.
Ortho-, Meta-, and Para-Directing Ability
Let’s say that one would like to add a functional group to a given molecule. To design an experiment to fix the correct functional group to a given molecule, one must have a deep understanding of the regiochemistry that accompanies these reactions. To determine whether an electrophile will add ortho, meta, or para to a molecule akin to benzene, induction through sigma networks, resonance delocalization, aligning of pi systems, and many other factors must be considered. Mistakes are very often made in this facet of organic chemistry, given the number of locations a moiety can fix to.
Let’s take the nitration of phenol as an example. We have a deactivating, electronegative nitro group that wishes to attach to phenol, though where it attaches is the question. If one were to write out the three mechanisms (ortho, meta, and para), that this molecule can perform, a logical mechanistic reason for regiochemistry can be developed. The transitory arenium ion that forms from this mechanism will be more stable if it were added to the para, or ortho positions because the activation energy required to halt aromaticity will be smaller.
What some may easily forget, is that electron density will be more concentrated at the ortho and para positions. This fact becomes more apparent if one were to draw dipolar resonance forms, showing the negative charge placed at these positions.
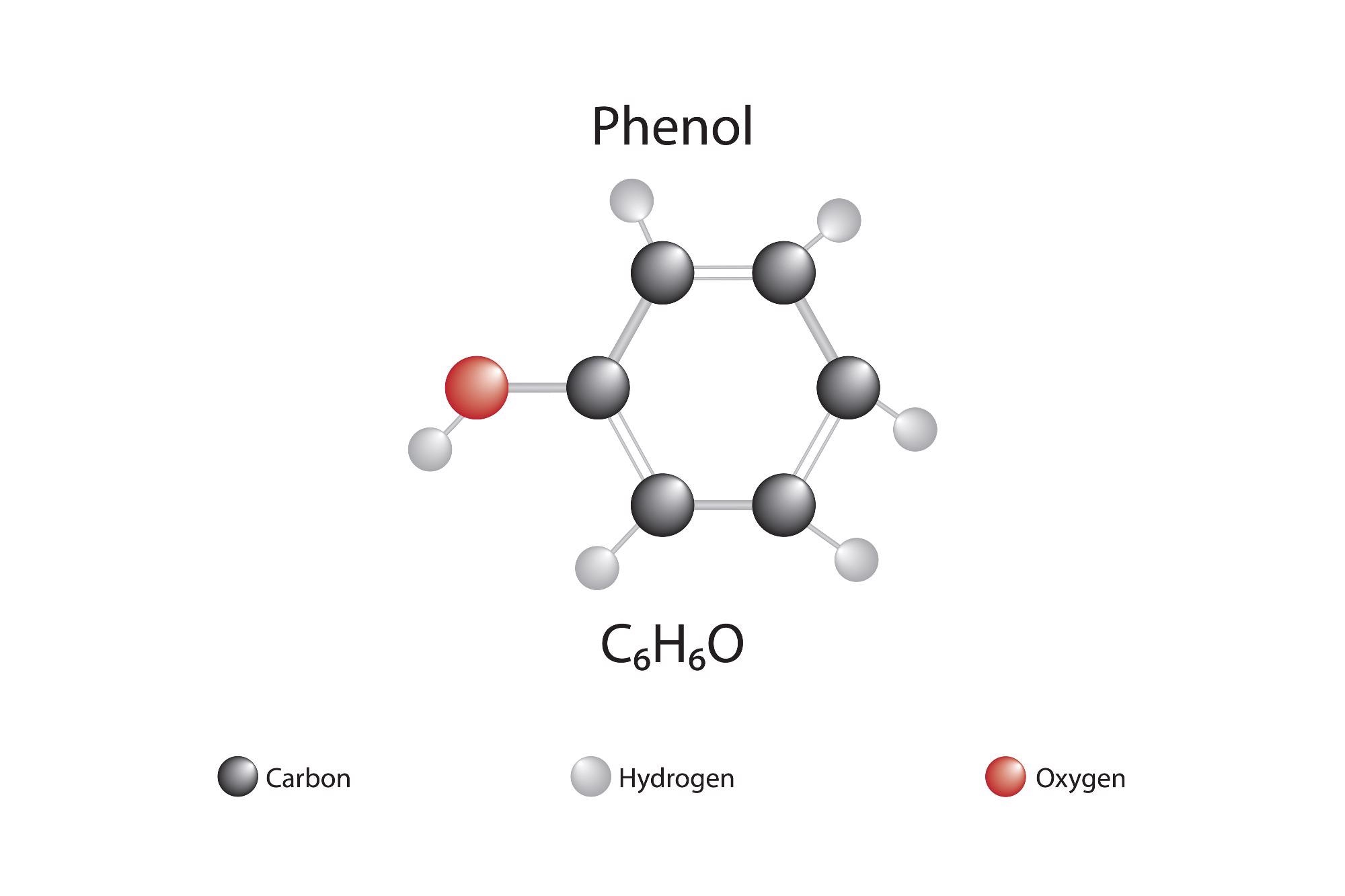
Image Credit: firatturgut/Shutterstock.com
Radical Reactions
Intermediates like carbocations, carbanions, and carbenes are commonplace when expanding upon organic chemistry. However, outside the scope of ozone layer damage, and polymer chemistry, radical reactions are sometimes overlooked. A free radical is an electron-deficient intermediate, though it is dissimilar to the carbocation in that it is a neutral species. This lone electron resides in the p-orbital and can be stabilized by sigma donation.
The reason that most students studying organic chemistry find this practice enigmatic is because there is a surfeit of different reactions the radical can perform once it is generated. Abstraction, disproportionation, β-cleavage, dimerization, thermolysis, and simple addition are just some of the mechanism classifications these radicals can undergo. What’s more, when most scientists discuss radical reactions, these intricate mechanisms play a part in generating just one simple molecule or polymer.
Radical reactions commence with initiation (most often thermolysis plus abstraction), followed by propagation via addition and abstraction, ending with a termination reaction that can encompass many different mechanisms, like dimerization for example. Many mistakes can be made when designing an experiment involving radicals. Not only do you have these intermediate reactions to take into consideration, but you must also harbor a keen awareness of what your reagent concentrations are. For a practical reaction to take place, neutral reagents must be in high concentrations, while radical intermediates are kept to a minimum, reducing the odds of termination steps occurring too frequently.
The practice of organic chemistry deals with the expounding of carbon-containing molecules, assaying their composition, reaction properties, and overall structure. Carbon-based life and carbon chemistry, in general, is extremely versatile, and rare in their complexity. So much so in fact that all chemistry is often divided into organic and inorganic, attributing to its prominence. For these reasons, one must proceed with caution when mapping out reactions in an experimental setting.
Sources:
- Nilapwar SM, Nardelli M, Westerhoff HV, Verma M. (2011) Absorption spectroscopy. Methods Enzymol. 2011;500:59-75
- Silvarajoo, S., Osman, U. M., Kamarudin, K. H., Razali, M. H., Yusoff, H. M., Bhat, I., Rozaini, M., & Juahir, Y. (2020). Dataset of theoretical Molecular Electrostatic Potential (MEP), Highest Occupied Molecular Orbital-Lowest Unoccupied Molecular Orbital (HOMO-LUMO) band gap, and experimental cole-cole plot of 4-(ortho-, meta- and para-fluorophenyl)thiosemicarbazide isomers. Data in brief, 32, 106299.
- Kokel, A., Schäfer, C., & Török, B. (2019). Organic Synthesis Using Environmentally Benign Acid Catalysis. Current organic synthesis, 16(4), 615–649.
- Nieves-Quinones, Y., & Singleton, D. A. (2016). Dynamics and the Regiochemistry of Nitration of Toluene. Journal of the American Chemical Society, 138(46), 15167–15176.
- Wang, S. C., & Frey, P. A. (2007). Binding energy in the one-electron reductive cleavage of S-adenosylmethionine in lysine 2,3-aminomutase, a radical SAM enzyme. Biochemistry, 46(45), 12889–12895.
- Pham-Huy, L. A., He, H., & Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. International journal of biomedical science : IJBS, 4(2), 89–96.
Further Reading