Through the work of two research labs, we know now that mammalian tissues express two cannabinoid receptors CB1 and CB2 receptors.
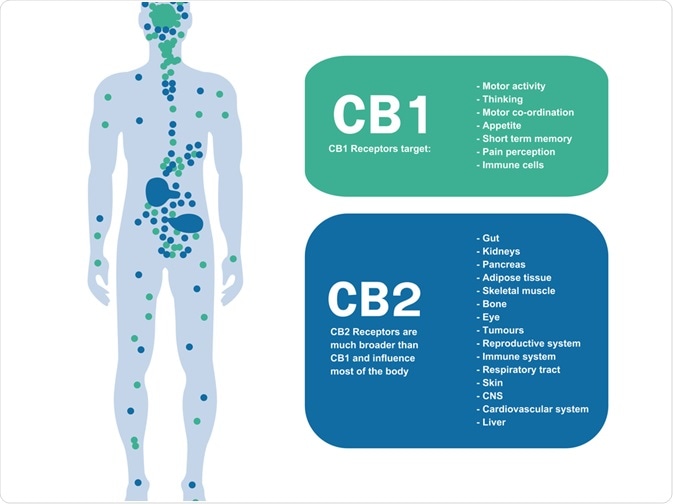
Image Credit: Shutterstock.com
These receptors are classed as G protein-coupled receptors (GPCRs) therefore coupled to Gi/o proteins, negatively to adenylate cyclase and positively to mitogen-activated protein kinase (MAPK). CB1 proteins are coupled through these Gi/o proteins to ion linked channels.
CB1 receptors are typically expressed by non-neuronal cells and tissues inclusive of the pituitary gland, immune cells, and reproductive tissue.
Similarly, they are also found in the central and peripheral nerve terminals mediating the inhibition of transmitter release.
On the other hand, CB2 receptors are often expressed on immune cells due to their ability to modulate cytokine release. Therefore, one can ascertain these receptors have key roles in modulating the ongoing release of chemical messengers. They have also been detected on the central nervous system, primarily on microglial cells.
The expression pattern of CB1 receptors on the nervous system can be attributed to the significant effects of THC- the psychotropic component of cannabis. This can often be seen in its impacts of reduced motor activity, induction of analgesia and increased food intake.
Agonists
The expression of cannabinoid receptors on mammalian tissues was shortly followed by the discovery they can produce endogenous ligands in response to these receptors or ‘endocannabinoids’.
These endocannabinoids can likely function as both neuromodulators and immunomodulators. Indeed, there is evidence to suggest that they can serve as retrograde synaptic messengers.
There is early evidence to suggest that these endocannabinoids can be ‘autoreactive’ in pathological states where increased amounts have been produced in response to muscle spasm in multiple sclerosis or inflammatory pain, in turn, these molecules can ameliorate such symptoms.
Therefore, the endocannabinoid system poses an attractive pharmacological target through their roles in synthesis, membrane transport and metabolism of endocannabinoids- as these have been shown to have therapeutic potential.
The discovery of new pharmacological agonists relies upon the availability of suitable bioassays. Concerning CB1 receptors, the most common in vivo study is the mouse tetrad, in which their ability to produce hypokinesia, hypothermia, catalepsy in the Pertwee ring test and antinociception in the tail-flick or hot plate test is determined in the same animal. Currently, there is no standard in vivo assays for CB2 receptors.
Whilst there are several in vitro assays for both receptors that involve the use of membrane or tissue receptors expressed through transfection. At this point, further assays such as radiolabelling can determine the binding capabilities of test compounds to displace a cannabinoid receptor ligand.
In addition to this, functional in vitro assays have been used to determine the effects of compounds on downstream signaling of the receptors such as the elevating of intracellular free calcium.
Antagonists
Alongside the discovery of the CB1 and CB2 receptors was the development of selective antagonists of each receptor.
Among these are the CB1 selective SR141716A and AM251. These have been demonstrated to produce inverse cannabimimetic effects in CB1 assay systems, that are opposite in direction from those produced by agonists for these receptors.
In vivo inverse effects of SR141716A in mice show hyperalgesia in neuropathic pain models; increased intestinal motility and suppression of food consumption.
Meanwhile, in vitro effects demonstrate enhancement of an ongoing release of acetylcholine and noradrenaline. Nonetheless, there is evidence to suggest that these effects are not seen through a single mechanism. Some of these effects of ligands such as SR141716A are produced in the absence of any detectable ongoing endocannabinoid release.
This occurs in systems where CB1 receptors have been genetically inserted, and so are usually overexpressed, and in systems in which these receptors are expressed naturally. Some of these inverse effects are likely induced by a process of ‘inverse agonism’ in which CB1 receptors are shifted from a proposed constitutively active ‘on’ state to one or more constitutively inactive ‘off’ states.
This putative mechanism relies on the assumption that CB1 receptors can exist in a constitutively active state in which they undergo some degree of spontaneous coupling to their effector mechanisms even in the absence of an endogenously released or exogenously added agonist
The selective CB2 selective antagonist SR144528 produces inverse cannabimimetic effects in some basic receptor containing assays. However, the mechanism underlying the action of these compounds is yet to be determined.
Therapeutic potential
Marinol is an oral preparation of tetrahydrocannabinol (THC) and nabilone and is a synthetic analog currently licensed for clinical use in some countries as both appetite stimulants and antiemetics.
CB1 receptor agonists have other potential uses inclusive of glaucoma management, pain, a certain subset of cancers and various pathologies involving motor dysfunction e.g. multiple sclerosis or spinal injury.
The key area in preclinical, clinical and anecdotal data is the betterment of tremors, pain and muscle spasms through spinal injury and multiple sclerosis. Indeed, recently a THC extract has been developed as a medicine for the management of multiple sclerosis.
Whilst there has been little development of CB2 agonists as a therapeutic field, early evidence indicates it might have the potential to relieve inflammatory and neuropathic pain. Yet, these are to be identified and validated as a target.
References:
- Howlett, A.C., et al., Pharmacol. Rev., 2002. 54: p. 161-202.
- Pertwee, R.G., Life Sci., 2005. 76: p. 1307-1324.
- De Petrocellis, L., M.G. Cascio, and V. Di Marzo, Br. J. Pharmacol., 2004. 141: p. 765–774.
- Pertwee, R.G., Br. J. Pharmacol., 1972. 46: p. 753-763.
- Pertwee, R.G., Pharmacol. Ther., 2002. 95: p. 165-174.
Further Reading
Last Updated: Mar 18, 2020