CRISPR (clustered regularly interspaced short palindromic repeats) is a genre of DNA sequences found in prokaryotes from bacteriophages that had previously invaded the species or strain. With Cas (CRISPR associated) proteins it provides an anti-viral defense for prokaryotic cells.
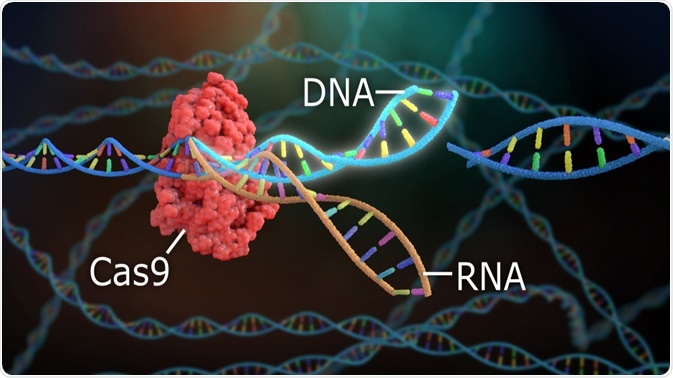
Image Credit: Nathan Devery/Shutterstock.com
A simplified version of this defense system is used as a genetic engineering tool. This allows scientists to cut an organism’s DNA at a specific location and genes added or removed from the genome.
Therefore, this system has heavy implications in biomedicine due to the ability to knock out genes associated with a disease or add genes that a person may be missing. But there are also ethical issues surrounding the limits of acceptable use for CRISPR-Cas and human intervention in nature.
What is CRISPR-Cas9?
As a prokaryotic defense mechanism CRISPR RNA (crRNA) associates with Cas proteins. Most notable is Cas9, the DNA endonuclease in type II systems. The crRNA guides the Cas proteins to the target site on the viral DNA, which is degraded, preventing the viral invasion of the cell.
Unlike in type I and III systems which only require crRNA, Cas9 requires both crRNA and trans-activating crRNA (tracrRNA). It partially complements pre-crRNA and therefore forms an RNA duplex, which guides Cas9 to the target site.
CRISPR gene editing uses a simplified version of this crRNA-tracrRNA-Cas9 system. Through synthesis of the RNA component, the complex is targeted to a specific DNA sequence where it cuts the DNA and can add or remove bases. Homology-directed repair in which a strand of DNA is delivered alongside the RNA-Cas9 complex, changes as small as a single base pair could theoretically be made.
The use of CRISPR is most clear in treating simple genetic diseases. For example, cystic fibrosis is caused by mutations in the CTFR protein leading to various long-term complications for the patient. Due to the ineffectiveness of conventional medicine, the potential for CRISPR in this disease is exciting.
In pre-clinical studies, CRISPR has been shown to correct common mutations in a human stem cell line derived from cystic fibrosis patients. There is hope that this could be converted to clinical trials before long.
There is also hope that this system could be used in persistent viral infections, such as HIV, to break down viral DNA. In complex diseases, there is potential for CRISPR to be a useful tool but a deep understanding of the genetic causes of disease is required.
Another question is the in vivo delivery of CRISPR therapies and how to target it to the cell of choice without causing wide-scale changes. Where the target sequence has identical twins in other loci of the genome would also pose significant problems in the delivery of any therapy.
New species and ethical concerns
An important concern with CRISPR is how a gene change will affect an organism as a whole. The link between a gene and biological phenotypes are rarely, if ever, completely understood. Even in a simple monogenic disease, it would be unknown how changes to the genome would affect its interactions with other molecules in the cell.
This would be a particular area of worry if CRISPR was used in more complex diseases. It would be impossible to predict all the interactions changes to multiple genes would affect. The risk or benefit to an individual would be difficult to assess due to these factors.
Using CRISPR scientists can create new single cellular organisms relatively simply. Changing a few genes in a small organism with a small genome would allow someone to form a new species, closely related to an existing bacterium, fungus, algae, etc.
This by itself would raise ethical questions surrounding the effect this organism would have on its immediate environment and the knock-on effect of this. But this equally could provide an opportunity in a variety of fields.
In humans, while the use of CRISPR in somatic cells has some ethical concerns, it is unlikely that there would be knock-on effects on the individual’s progeny, particularly in non-stem cell therapies. For example, if theoretically cystic fibrosis was cured in the individual, their germline cells would still carry the faulty DNA.
Using CRISPR to edit germline cells has significantly more ethical questions. Any mistakes in gene editing would affect not only the individual but also any offspring they may have, increasing the potential risk of any such therapy.
In its medical use, these concerns are legitimate but the overall aim to reduce the suffering of a person with a chronic, genetic disease is fair. The potential use of CRISPR in non-medical instances, for example, to select ‘good’ biological phenotypes raises questions of human vs. nature, developing international legislation may be essential to set limits to the use of CRISPR in the future.
References
- Brokowski, C. and Adli, M. (2019) ‘CRISPR Ethics: Moral Considerations for Applications of a Powerful Tool’, Journal of Molecular Biology. doi: 10.1016/j.jmb.2018.05.044.
- Redman, M. et al. (2016) ‘What is CRISPR/Cas9?’, Archives of Disease in Childhood: Education and Practice Edition. doi: 10.1136/archdischild-2016-310459.
- Wiles, M. V. et al. (2015) ‘CRISPR–Cas9-mediated genome editing and guide RNA design’, Mammalian Genome. doi: 10.1007/s00335-015-9565-z.
Further Reading