When you say "electrolysis" to consumers, they probably think of hair removal, not that electrolysis is also a fundamental chemical and manufacturing process. It is a technique that uses a DC electrical current to drive an otherwise non-spontaneous chemical reaction. In other words, the energy for chemical decomposition is a current passing through a liquid solution containing ions (known as an electrolyte). Electrolysis has many important applications. Reverse Electrolysis is the technology employed by batteries in the energy business.

Image Credit: Macrovector/Shutterstock.com
Martinus van Marum accidentally discovered Electrolysis while attempting to reduce tin, zinc, and antimony using salts in his electrostatic generator in 1785. Over the following years, various people, including Sir Humphry Davy, worked on Electrolysis. In 1834 Michael Faraday introduced the term "Electrolysis" and published his two laws of Electrolysis which allowed a mathematical expression of the process. He also used the terms electrolyte, anode, cathode, anion, and cation for the first time.
Faraday's first law of Electrolysis is that the mass{\displaystyle m} of elements deposited at an electrode is directly proportional to the charge.{\displaystyle Q}
Faraday's second law of Electrolysis is that if the same amount of electric current passes through different electrolytes/elements connected in series, the mass of the substance liberated/deposited at the electrodes in grams is directly proportional to their chemical equivalent weight.
What is an Electrolysis Cell?
Many of us will remember seeing a typical electrolysis cell in School Science lessons, it consists of two electrodes, called an anode, and a cathode, immersed in an electrolyte. An electrical circuit also connects the two electrodes, and a current passes through the system by applying a voltage. The setup is almost identical to a battery, except that no voltage is applied to the electrodes. In a battery, electric current is delivered by the reactions at the electrodes to the circuit instead.
The electrolyte is a solution of an ionic compound in a solvent that will produce mobile ions or a substance that contains ions that are free to move around (like an ionic conducting polymer). The electrode will attract ions with the opposite charge. So positive cations are attracted to the negative cathode, and negative anions are attracted to the positive anode. A negatively charged ion will migrate to the anode, give up one or more electrodes, and become neutral or positive.
Vice versa, a positively charged ion will receive one or more electrons at the cathode and become neutral or negatively charged. An applied voltage drives the reaction in an electrolytic cell. The minimum voltage required to drive the reaction is called the "Decomposition potential."The electrons given up at the anode will travel through the circuit as an electric current in a battery.
The electrolysis process is an oxidation/reduction reaction. The loss of electrons is an oxidation reaction, and the gain of electrons is a reduction reaction. The electrodes are often graphite, platinum, or other non-reactive substance. The choice of material depends on the reactivity of the electrolyte.
The process of interchanging ions will often lead to a change of state, for example, from liquid to gas, deposition or precipitation of a solid, etc. In systems with metallic salt electrolytes, reduced metal from the electrolyte deposits at the cathode. This process is known as electroplating or electrorefining. Electrolysis is used for refining and purifying Aluminium, Lithium, Sodium, Potassium, Magnesium, Calcium, Copper, and other metals. The process is also used in the manufacture of perfluorinated organic compounds. It is also possible to grow specialized crystals for the electronics industry electrolysis.
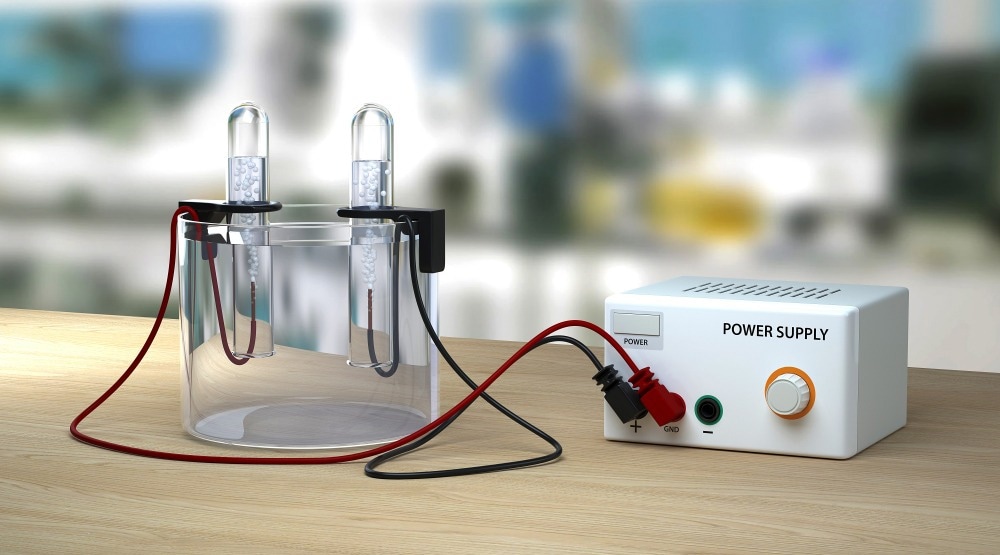
Image Credit: haryigit/Shutterstock.com
Chloralkali Process
One of the most important processes in the Chemical Industry is the Chlor-Alkali process. This process is an application of Electrolysis that produces sodium hydroxide, Hydrogen, and Chlorine which are significant components of the chemical industry. The reactions are:
2NaCL + 2H2O → 2NaOH + H2 + Cl2
The anodic reaction is: 2Cl- → Cl2 + 2e-
The cathodic reaction is: 2H20 + 2e- → H2 + 2OH-
If the gases produced are not removed, they will reform the original constituents. One of Britain's most significant industrial complexes, the Ineos site at Runcorn, uses this process as a basis for its manufacturing.
The Nernst equation can calculate the minimum voltage required to drive this type of reaction. Usually, a small extra voltage, known as an over-voltage, is applied to improve the process.
Other Uses for Electrolysis
Electrolysis is used for refining and purifying Aluminium, Lithium, Sodium, Potassium, Magnesium, Calcium, Copper, and other metals. The manufacture of perfluorinated organic compounds uses Electrolysis. It is also possible to grow specialized crystals for the electronics industry electrolysis
Electrolysis is used extensively in water treatment for the production of purified water. The Electrodeionisation process is a sophisticated process that uses electrodes, ion exchange resins, and ion exchange membranes. It is typically used as a polishing process to remove any remnants of inorganic and organic ionic substances in producing ultra-pure water in laboratories, medicine, industry, and pharmaceutical manufacturing.
Electrolysis has excellent potential in the fight against climate change, and it is a significant component in producing "Green Hydrogen." Currently, 95% of Hydrogen manufacture is by splitting natural gas, releasing substantial amounts of carbon dioxide, whereas splitting water into its component elements using renewable energy produces zero carbon dioxide. The process is not presently cost-competitive. The National Renewable Energy Laboratory in the USA estimates that 1kg of Hydrogen has the equivalent energy of 3 kg of petrol. Burning Hydrogen produces water vapor as its waste which is carbon neutral. Many research programs are now looking at hydrogen production by Electrolysis of coal slurry, alcohols, and other organic solutions. Some programs also look at electrolytic applications in biological processes to produce Hydrogen. These programs aim to reduce the unit cost of "Green Hydrogen."
Energy storage systems like redox flow batteries are suitable for large batteries up to utility-scale use Electrolysis. The components or redox flow batteries have much lower environmental footprints than the more well-known Lithium batteries (which run on a reverse electrolysis process). Redox flow batteries are made from more common minerals and are widely and inexpensively available with fewer ethical side effects.
Sources:
- Clean Hydrogen from water electrolysis: research into catalysts to meet global targets
- May 27, 2021, by Glenda Chui energypost.eu/.../
- Lignin_Assisted Water Electrolysis for Energy Saving Hydrogen Production with Ti/PbO2 as the Anode https://www.frontiersin.org/articles/10.3389/fenrg.2021.762346/full
- Electroctalytic carbon dioxide reduction: from fundamental principles to catalyst design , Xiaolong Zhang, Si-Xuan Guo, Karl A. Gandionco, Alan M. Bond, Jie Zhang https://www.sciencedirect.com/science/article/pii/S2590049820300217
- Renewable Electricity Storage using Electrolysis, Zhifei Yan, Jeremy L. Hitt, John A. Turner, and Thomas E. Mallouk https://www.pnas.org/content/117/23/12558
Further Reading