The following article has been adapted from a poster by TCG Life Sciences presented at ELRIG UK Drug Discovery Conference 2023.
Introduction
According to the World Health Organization (WHO), approximately two million children under five years of age die in sub-Saharan Africa and Asia from one of the following six diseases: pneumonia, diarrhea, malaria, meningitis, HIV, or measles.
Despite substantial work (and Nobel Prize in 2015 in Physiology and Medicine) in anti-malarial research over the past years, malaria remains a major health and economic burden. In view of the key milestones set by the WHO, such as a tenfold reduction in malaria incidence and deaths by 2030 (compared with 2015), the discovery of new malaria medicines is a key pillar in reducing drug-resistant malaria-associated deaths by this margin.
Background
Significant scientific progress has indeed been made in anti-malarial research over the last two decades. However, P. falciparum remains the most lethal human pathogen.
In 2017, about 219 million malaria cases were reported.,including nearly 435,000 deaths worldwide by P. falciparum (61% of which were children under 5 years of age). Amongst the current medicines, the use of artemisinin-based combination therapies remains to be the gold standard. It has contributed to a global reduction of malaria-related deaths around the world by more than 40% over the last decade. Despite these efforts, signs of resistance to artemisinin have emerged.
Therefore, new, affordable and safe drugs are required to tackle malaria, either as a single entity or in combinations as appropriate. Each intervention will eventually meet one or a number of different target candidate profiles, such as:
- Treal vulnerable populations.
- Interrupt the parasite life cycle by blocking transmission to the vectors that need just one administration.
- Prevent infection or target malaria species that transiently remain dominant in the liver.
Fast-acting and can maintain active plasma concentrations for a long period upon single dosing p.o. In this context, identifying potent, orally available anti-malarial agents, either acting via known or with new mechanisms of action against resistant strains of the parasite P. falciparum (possibly acting against different life cycle stages) and also with acceptable pharmacokinetic parameters are the objectives of this presentation.
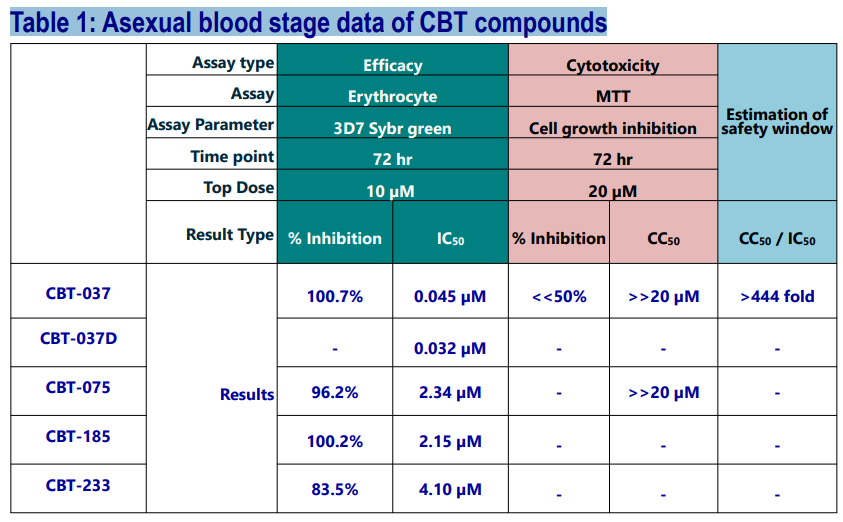
In vitro Anti-Malarial Activity of CBT Compounds
Four structurally distinct Hit compounds have been envisaged via a ligand-based approach and synthesized. CBT-037 was obtained after a few iterations of this specific scaffold. For a head-to-head comparison, its novel deuterated analog was also synthesized and tested.
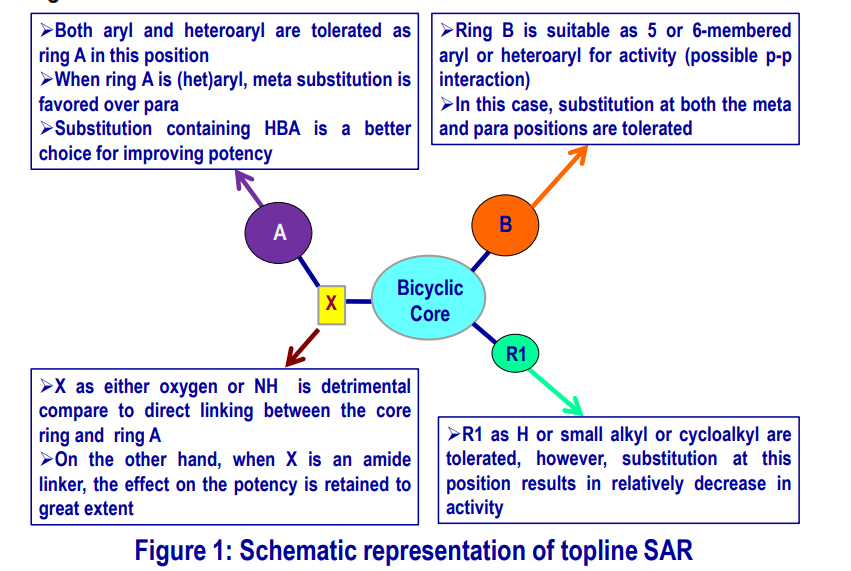
Topline SAR of CBT-037 Scaffold
From the analogs synthesized in this series, a brief SAR can be drawn that has been schematically represented in the diagram below.
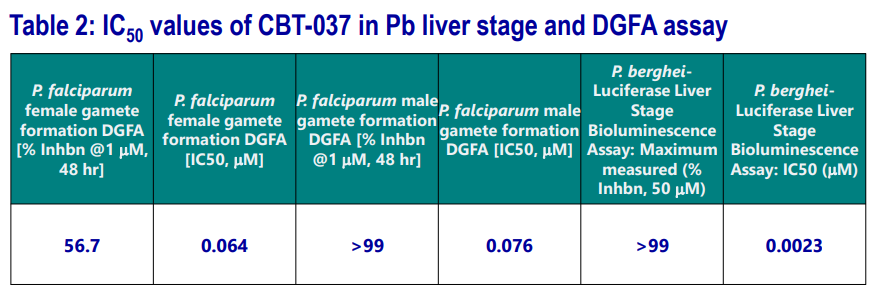
Activity of CBT-037 in other Lifecycle Stages
CBT-037 was explored in extensive studies and found to be active in both Pb-liver stage as well as in dual gametocyte formation (DGFA) assays.
ADME and hERG Binding Data of CBT-037
Tier 1, 2 ADME profiling (stability against different liver microsomes, hepatocytes; CYP inhibition studies, solubility, permeability, plasma�protein binding) along with hERG binding liability of CBT-037 were carried out. The results are summarized in the following table.
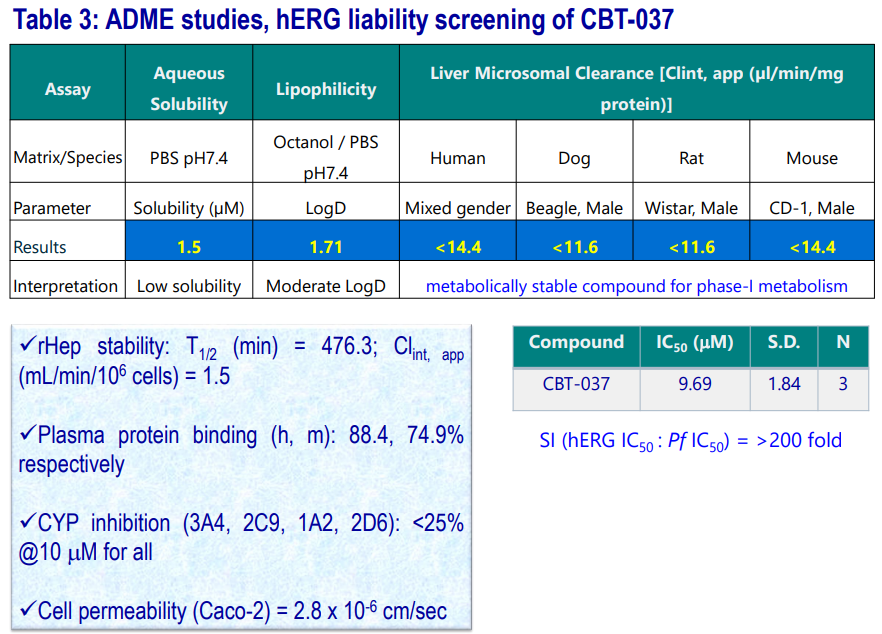
- Compound is metabolically stable (T1/2 : >120 mins) in various liver microsomes.
- Showed significant stability in rat Hepatocytes.
- Renders low aqueous solubility at pH7.4
- Has moderate permeability.
- Compound is devoid of DDI and displays good PPB parameters.
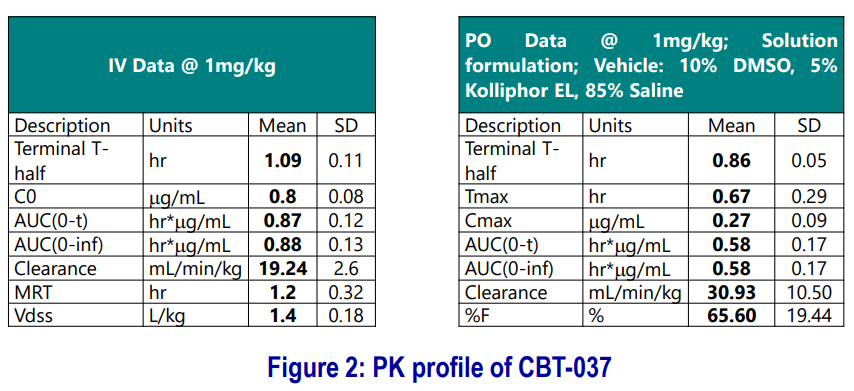
CBT-037: PK and In vivo Efficacy
- PK studies were carried out in mice (n=3) following IV and PO dosing.
- Preliminary formulation optimization was carried out using four different formulations (see example below).
The compound was tested against P. falciparum in a humanized mouse model, 4-days dosing, QD, per oral. Formulation 10% DMSO, 5% CremEL (Kolliphor EL), 85% Saline was used.
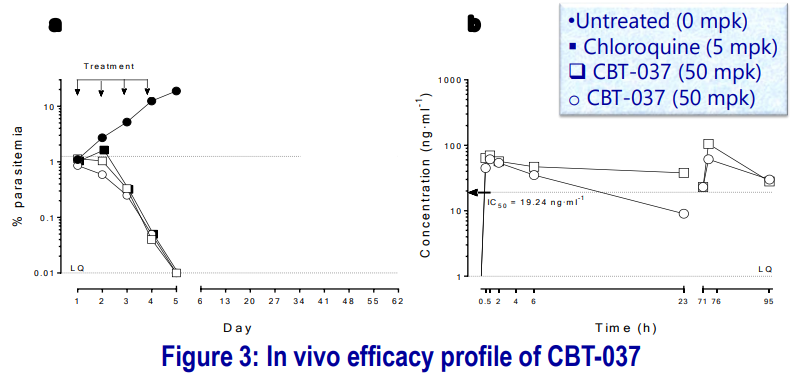
- Parasitemia is expressed as % of infected human erythrocytes with respect to total circulating erythrocytes (murine + human). The compound was efficacious against P. falciparum after four days of treatment at 50 mg-kg-1.
- The average drug concentration during the assay (0-95 h) was above the IC50 Concentration.
- CBT-037 demonstrated a reduction in % parasitemia even after 1st dosing. The mice were kept to test recrudescence: at day 15 of the assay, there were no detectable parasites (to be checked on longer day/s).
Conclusions
Herein, we disclosed the identification of four different series as anti-malarial Hits. Out of those, CBT-037 was found to be a promising lead molecule for further development, and TCGLS is keenly interested in collaboration opportunities.
Acknowledgments
TCG LS Management is gratefully acknowledged for the opportunity to do this work. Sincere thanks to Dr. B. Laleu, Dr. F.Escudie and MMV Management for the valuable discussions, guidance and offering of support for the in vitro and In vivo anti-malarial studies. Thanks are also due to Malay S, Mithun M, Tarun M, TCG compound management, analytical and logistic teams.
Selected References
- [1] a. World Malaria Report 2017: World Health Organization: Geneva, Switzerland; b. Sachs, J.; Malaney P, Nature, 2002; 415:680; c. Wells TNC, Hooft van Huijsduijnen R, Van Voorhis WC, Nat. Rev. Drug Disc. 2015; 14:424–442.
- [2] Cabrera DG, Horatscheck A, Wilson CR, Basarab G, Eyermann CJ, Chibale K, J. Med. Chem. 2018; 61(18):8061–8077.
- [3] Burrows JN, Duparc S, Gutteridge WE, Hooft van Huijsduijnen R, Kaszubska W, Macintyre F, Mazzuri S, Möhrle JJ, Wells TNC, Malar. J. 2017; 16:26
Last Updated: Feb 11, 2025