Tubular organs refer to the body’s hollow, tube-like structures, which are vital for critical bodily functions such as blood circulation (via blood vessels), digestion (via the intestines), as well as breathing (via the trachea and bronchi). Such tubular tissues and tissue structures are also crucial for sustaining essential processes in human health, such as nutrient absorption and oxygen transport.1
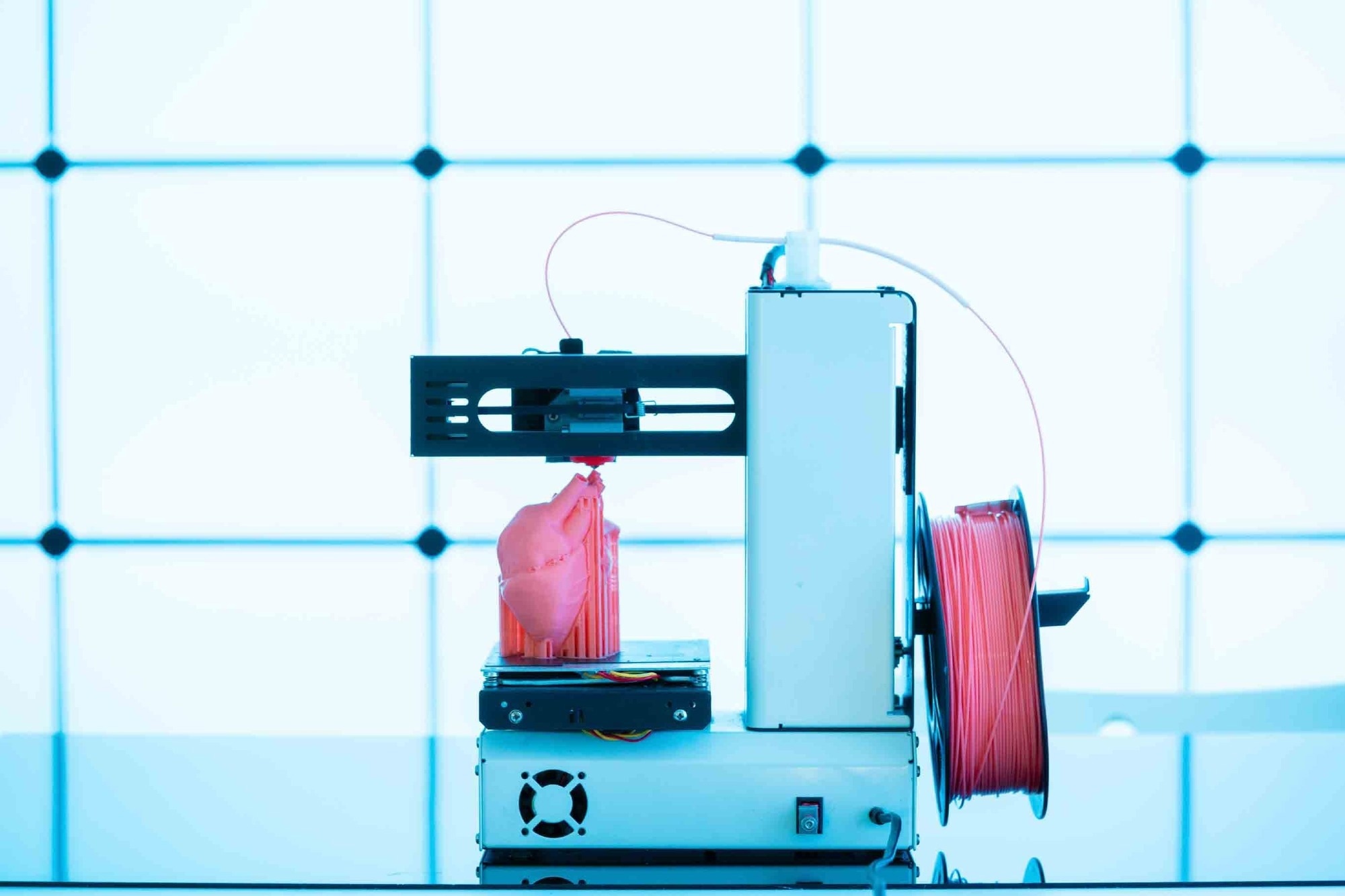 Image Credit: luchschenF/Shutterstock.com
Image Credit: luchschenF/Shutterstock.com
Introduction
Given the susceptibility of these tubular systems to disease and trauma, much research has been focused on the engineering of tubular organs, using techniques from tissue engineering and regenerative medicine, thereby seeking to develop functional organ replacements for those that are damaged or failing. With this medical innovation, it serves as a viable alternative to conventional organ transplants, which are hindered by limited donor availability and immune rejection issues. 1-3
Moreover, patient-specific solutions are addressed in tubular engineering through the use of advanced technologies, such as 3D bioprinting and tissue scaffolding. These technologies allow for the customization of vascular grafts to match the individual patient’s anatomy and physiological needs.2 This personalization minimizes complications and enhances graft integration, promoting more successful long-term outcomes.
Discover more about biotechnology
Technologies and Techniques for Tubular Organ Engineering
Tissue Scaffolding
One of the fundamental techniques in tubular organ engineering is the use of tissue scaffolding. Biodegradable scaffolds are designed to imitate the natural structure of tubular organs, providing a temporary framework that supports the attachment and growth of cells. Over time, as cells proliferate and form functional tissues, the scaffold gradually degrades, leaving behind the newly formed organ without any foreign materials.4,5
3D Bioprinting
3D bioprinting, also known as additive manufacturing, is a cutting-edge technology used to fabricate tubular organs with high precision. It often involves either extruding material through a nozzle or using photo-crosslinking from a liquid precursor. In extrusion-based printing, a liquid material is dispensed from a nozzle onto a platform, creating a single layer, whereby after the solidification of that layer, additional layers are applied to form a 3D object. By carefully controlling the placement of these materials, 3D bioprinting allows for the creation of complex, functional tubular organs that mimic the natural architecture and function of the body.1
Stem Cell Therapy
Stem cells play a crucial role in tubular organ regeneration due to their ability to differentiate into various types of cells required for functional tissues. In regenerative medicine, stem cells are introduced into the scaffolds or damaged areas, where they grow and differentiate into the necessary cell types, such as endothelial cells for blood vessels or epithelial cells for airways, thereby aiding in the repair or creation of tubular organs.6
Decellularisation and Recellularisation
Another important technique in tubular organ engineering is decellularisation and recellularisation. In this process, a natural organ is decellularised, which removes all of the original cells, leaving behind only the extracellular matrix, which acts as a biological scaffold. The resulting scaffold can then be recellularised with the patient’s cells, reducing the risk of immune rejection while creating a functional organ that closely mimics the original tissue's properties.1,7,8
Applications in Regenerative Medicine
Vascular Grafts
Engineered blood vessels, or vascular grafts, are being explored in cardiovascular treatments, such as bypass surgeries and vascular disease therapies. These grafts are designed to restore blood flow in patients suffering from blocked or damaged arteries. As reported by the University of Edinburgh, researchers from their School of Engineering, in collaboration with Heriot-Watt University, used a 3D printer with a rotating spindle to create tubular grafts from a water-based gel.9,10
These grafts were then reinforced using electrospinning, which produced nanofibers coated in biodegradable polyester. Testing revealed that the synthetic vessels are as strong as natural ones and can be produced in diameters ranging from 1 to 40 mm for diverse applications. In addition, their flexibility enables smooth integration into the body.9
Urinary and Digestive Systems
Significant advancements have been made in engineering tubular organs for the urinary and digestive systems. In the urinary system, bioengineered ureters and bladders can be used to replace damaged tissues. In contrast, in the digestive system, efforts are being focused on creating tissue-engineered intestines to treat conditions such as short bowel syndrome. 11,12
Respiratory System
Progress is also being made in the engineering of tubular structures for the respiratory system, such as the trachea. Bioengineered tracheas have the potential to revolutionize treatments for airway diseases and conditions, for example, tracheal stenosis, by offering biocompatible, functional replacements that can restore normal airflow in patients. 13
Find out more about stem cells
Challenges in Tubular Organ Engineering
Structural Complexity
One of the largest challenges in tubular organ engineering is replicating the complex structures and functions of natural organs. Tubular organs require specific mechanical properties, including flexibility and strength, to perform their functions effectively. Additionally, ensuring proper vascularisation and nutrient delivery within the engineered organ is critical to its long-term viability.14
Integration with the Host
Successfully integrating engineered organs with the patient’s body is another major challenge. The organ must not only function properly, though also avoid triggering immune responses that could lead to rejection. 15
Scaling for Clinical Use
Scaling up the production of engineered organs to meet clinical demand is a significant hurdle. Key challenges include inducing vascularisation, modulating the immune response, developing universal donor cell lines, and ensuring tissues replicate the in vivo environment with proper mechanical properties.16
In addition to the technical challenges of producing larger quantities of complex tissues, regulatory approval processes must be navigated to ensure safety and efficacy.17 Overcoming these barriers will be essential for bringing engineered tubular organs into widespread medical practice.
Impact on Healthcare and Organ Transplantation
Advances in tubular organ engineering have the potential to transform healthcare by alleviating the organ donor shortage and significantly lowering transplant waiting times. This technology could lead to improved patient outcomes by providing engineered organs as an alternative to traditional transplants.18,19
Additionally, engineered tubular organs could enable more personalized treatment options tailored to individual patients. It would improve compatibility and lower the risk of immune rejection, offering a more effective and sustainable solution for transplant patients.20
Click here to learn more on bioprinting
Conclusion
The engineering of tubular organs offers transformative potential in regenerative medicine and organ transplantation by addressing donor shortages and improving patient outcomes.
Advances in tissue scaffolding, 3D bioprinting, and stem cell therapy are key to creating functional, patient-specific organ replacements. However, overcoming challenges in replicating organ complexity, ensuring biocompatibility, and scaling production is essential. Thus, continued research and collaboration are vital to bringing engineered organs into clinical settings.
References
- Boys AJ, Barron SL, Tilev D, Owens RM. Building scaffolds for tubular tissue engineering. Front Bioeng Biotechnol. 2020 Dec 10;8.
- Naegeli KM, Kural MH, Li Y, Wang J, Hugentobler EA, Niklason LE. Bioengineering human tissues and the future of vascular replacement. Circ Res. 2022 Jun 24;131(1):109-26.
- Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, et al. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int [Internet]. 2018 Jul 30;2018:1-24. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6091336/
- Krishani M, Shin WY, Suhaimi H, Sambudi NS. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels. 2023 Jan 23;9(2):100.
- Fukunishi T, Shoji T, Shinoka T. Nanofiber composites in vascular tissue engineering. In: Nanofiber composites for biomedical applications. 2017; p. 455-81.
- Jin Y, Li S, Yu Q, Chen T, Liu D. Application of stem cells in regeneration medicine. MedComm. 2023 Jun 17;4(4).
- Neishabouri A, Soltani Khaboushan A, Daghigh F, Kajbafzadeh AM, Majidi Zolbin M. Decellularization in tissue engineering and regenerative medicine: Evaluation, modification, and application methods. Front Bioeng Biotechnol. 2022 Apr 25;10.
- Ahmed E, Saleh T, Xu M. Recellularization of native tissue-derived acellular scaffolds with mesenchymal stem cells. Cells. 2021 Jul 15;10(7):1787.
- Artificial blood vessels could improve heart bypass outcomes [Internet]. The University of Edinburgh. 2024. Available from: https://www.ed.ac.uk/news/2024/artificial-blood-vessels-could-improve-heart-bypas
- Translational tissue-engineered vascular grafts: From bench to bedside. Biomaterials [Internet]. 2023 Nov 1 [cited 2023 Sep 19];302:122322. Available from: https://www.sciencedirect.com/science/article/pii/S0142961223003307?ref=pdf_download&fr=RR-2&rr=8093cc1a2ee7c356
- Yi XL, Lim D, Atala A, Yoo JJ. Engineering of the bladder and urethra. In: Reference Series in Biomedical Engineering. 2021; p. 259-84.
- Endo R, Sugimoto S, Shirosaki K, Kato H, Wada M, Kanai T, et al. Clinical challenges of short bowel syndrome and the path forward for organoid-based regenerative medicine. Regen Ther. 2023 Dec;24:64-73.
- Xu X, Shen Z, Shan Y, Sun F, Lü Y, Jun Z, et al. Application of tissue engineering techniques in tracheal repair: A bibliometric study. Bioengineered. 2023 Nov 6;14(1).
- Góral A, Pliszkal D, Mukherjee S, Ramakrishna S. Tubular tissues and organs of human body—Challenges in regenerative medicine. J Nanosci Nanotechnol. 2016;16:19-39.
- Sohn S, Buskirk MV, Buckenmeyer MJ, Londono R, Faulk D. Whole organ engineering: Approaches, challenges, and future directions. Appl Sci [Internet]. 2020 Jan 1;10(12):4277. Available from: https://www.mdpi.com/2076-3417/10/12/4277/htm
- Ghaemi RV, Siang LC, Yadav VG. Improving the rate of translation of tissue engineering products. Adv Healthc Mater. 2019 Aug 6;8(19):1900538.
- Three key hurdles restraining the growth of the tissue engineering field | Insights & events | Charles River Associates [Internet]. Crai.com. 2020 [cited 2024 Oct 7]. Available from: https://www.crai.com/insights-events/publications/three-key-hurdles-restraining-the-growth-of-the-tissue-engineering-field/
- Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: Solutions and opportunities. Int J Organ Transplant Med [Internet]. 2014;5(3):87-96. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4149736
- Dine A, Bentley E, Poulmarck LA, Dini D, Forte AE, Tan Z. A dual nozzle 3D printing system for super soft composite hydrogels. HardwareX. 2021 Apr;9:e00176.
- Lam EHY, Yu F, Zhu S, Wang Z. 3D bioprinting for next-generation personalized medicine. Int J Mol Sci [Internet]. 2023 Jan 1;24(7):6357. Available from: https://www.mdpi.com/1422-0067/24/7/6357
Further Reading
Last Updated: Oct 22, 2024