Ethics plays a significant role in society, with the actions in front of any scenario evaluated for being morally correct and ensuring a lack of harm. Within research and science, ethical standards ensure respect for an individual’s right to make informed decisions about participation.
The field of scientific research, which can utilize human participation, has a critical need for principles that prioritize standards of care and ensure the rights of others are upheld and respected, as well as being within the law.

Image Credit: marekuliasz/Shutterstock.com
Ethical Principles and Code of Conduct
The declaration of Helsinki, which comprises these ethical principles, was developed by the World Medical Association to guide physicians and participants of medical research involving human subjects.
This statement of duties is used to measure moral decisions that affect research participation within studies such as prospective and retrospective studies, cross-sectional surveys, focus groups, interviews, and any study that utilizes human biological samples, such as blood or sputum.
The Declaration of Geneva of the World Medical Association is also considered when it comes to ethical principles to ensure the health and well-being of patients is thought of as the first consideration. This is also reinforced by the International Code of Medical Ethics, which states, ‘‘A physician shall act only in the patient’s interest when providing medical care which might have the effect of weakening the physical and mental condition of the patient.’’
Ethics versus Interests of Science and Society
With the primary purpose of scientific and medical research involving the advancement of prophylactic, diagnostic, and therapeutic procedures as well as the understanding of disease pathogenesis, there may be times where the ‘greatest good for the greatest number’ can be thought to be more significant than prioritizing the health of a minority number of participants.
The challenge of always remaining ethical and moral and holding patient health to the highest of standards as opposed to critical research, which may harm a small minority, can be difficult to some, and therein lies in the role of ethical review committees.
Ethical review boards hold a critical role in ensuring research that is being proposed meets standards of care and does not infringe on the rights of others as well as being within the law. These committees can be utilized to ensure these regulations are carried out for studies, with external, non-biased members being chosen to approve the study prior to being started.
The priority of these members is to ensure all decisions involving human participants do not cross standards of care, with participant consent being taken, data protection being prioritized, and evaluating whether the research itself is ethical and in no way harmful to its participants.
The exceptions for ethics approval consist of studies that use anonymous patient data from another source, which the authors did not collect. However, honesty and openness are of significant value within science, so statements with reasons for ethical approval exemption would be required for scientific publications.
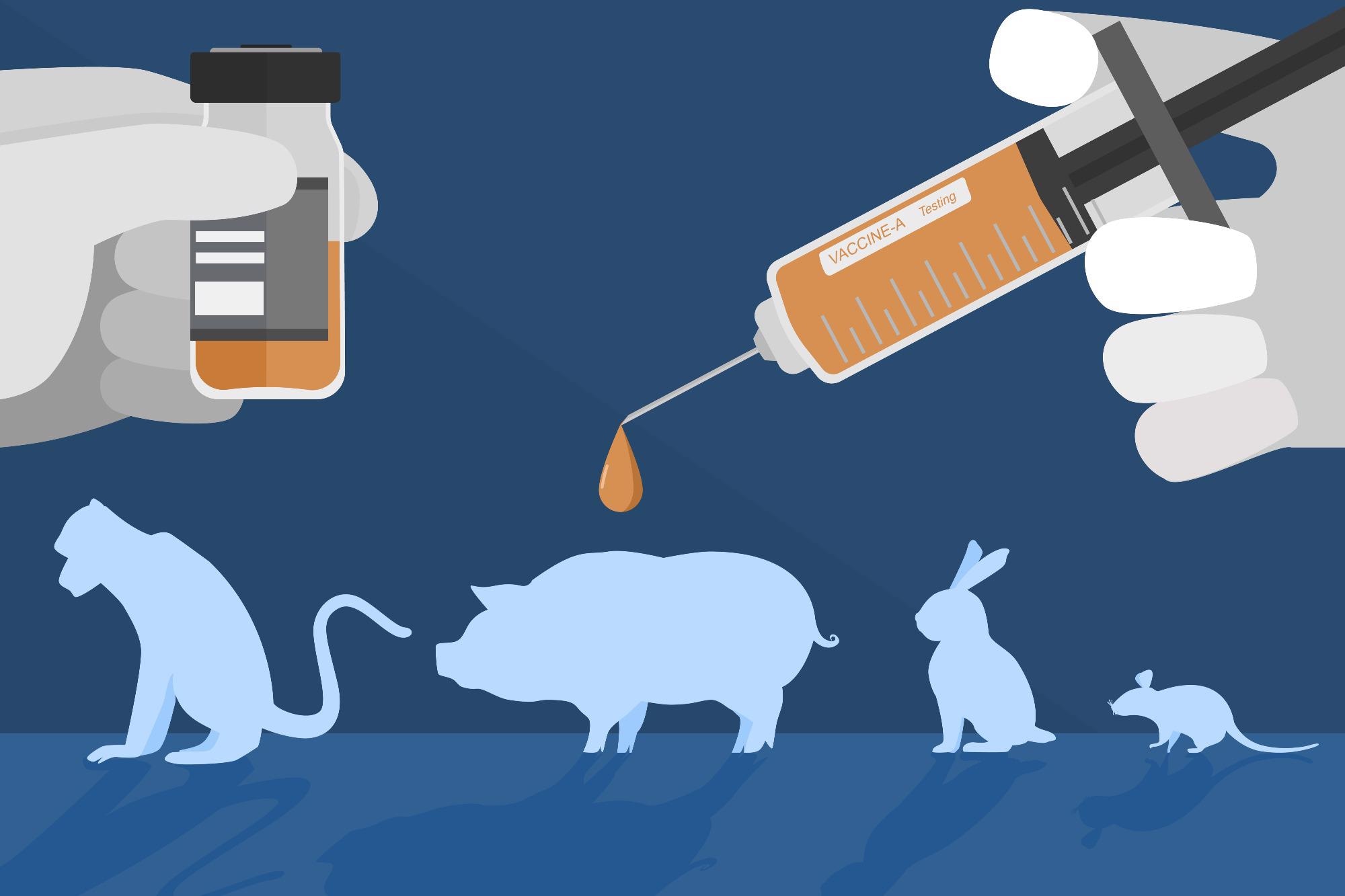
Image Credit: Hugethank/Shutterstock.com
Preventing Unethical Science
A famous example illustrating the requirement of ethical review committees comprises the researcher Edward Jenner, who tested smallpox on his son and other children in the neighborhood during early modern times. He is well-known for his innovative contribution to immunization. However, his research study and the inclusion of young children as participants can be seen as highly unethical.
Unethical science can include causing participants to undergo invasive procedures unnecessarily or further than required for the specific study. Other types of unethical science can include a disregard for ethical principles, including animal research. There are usually binding rules inspired by the Three Rs: Replacement, Reduction, and Refinement.
These principles ensure the necessity of the use of animals within research, with consideration for if the animal subjects or certain methods are required or can be replaced to eliminate harm. Additionally, utilizing the fewest number of animals possible and refining the experiment to minimize pain are also considered critical for animal experiments to be deemed acceptable for ethical approval.
The consideration of ethics for scientific decision-making is critical as the welfare and rights of individuals involved or impacted by all scientific decisions need impartial evaluation. These society rules ensure the balance and synergy between the advancement of science as well as the health and well-being of global populations.
Sources:
- Curzer, H., Perry, G., Wallace, M. and Perry, D., 2015. The Three Rs of Animal Research: What they Mean for the Institutional Animal Care and Use Committee and Why. Science and Engineering Ethics, 22(2), pp.549-565. Available at: DOI:10.1007/s11948-015-9659-8
- Riedel, S., 2005. Edward Jenner and the History of Smallpox and Vaccination. Baylor University Medical Center Proceedings, 18(1), pp.21-25. Available at: DOI:10.1080/08998280.2005.11928028
- Kim, W., 2012. Institutional review board (IRB) and ethical issues in clinical research. Korean Journal of Anesthesiology, 62(1), p.3. Available at: DOI:10.4097/kjae.2012.62.1.3
- Who.int. 2022. World Medical Association Declaration of Helsinki. [online] Available at: https://www.who.int/ [Accessed 9 February 2022].
- Wma.net. 2022. WMA - The World Medical Association-WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. [online] Available at: www.wma.net/.../ [Accessed 9 February 2022].
Last Updated: Oct 27, 2022