As of April 18th, 2021, roughly 142 million cases of the SARS-CoV-2 virus have been reported worldwide, while the mortality rate has just surpassed 3 million. Director of the National Institute of Allergy and Infectious Diseases (NIAID) Dr. Anthony Fauci asserts that our most generous forecasts predict herd immunity by the end of 2021. Yet, with vaccination rates dithering on account of transport, storage, and production, this future is still a long way away.
Other strains of coronavirus such as Severe Acute Raspatory Syndrome (SARS), and Middle East Respiratory Syndrome (MERS) have led to comparable pandemics on a much smaller scale. It is believed that the grade of biotechnology present in today’s labs may be able to influence the cellular machinery that the COVID-19 virus is so dependent on.
SARS-CoV-2 Virus Molecule. Image Credit: Orpheus FX/Shutterstock.com
The Physiology of the COVID Capsid
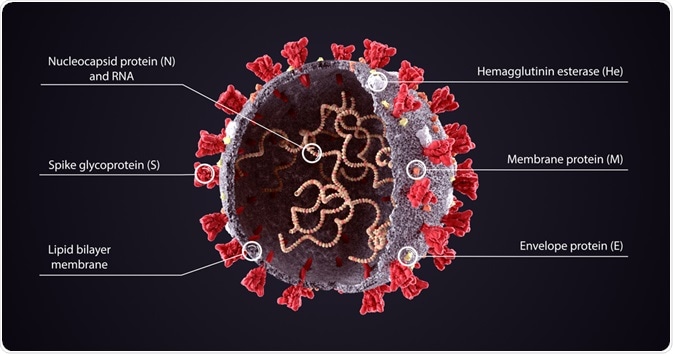
Coronaviruses constitute a variety of common viruses that are found in humans and animals. Condensing the pathological edifice of the COVID-19 capsid, we find that the integral proteins are the spike (S) protein, the envelope (E) protein, the membrane (M) protein, and the nucleocapsid (N) protein.
Spike proteins can be found smattering the extracellular side of the virus’s phospholipid bilayer, and are responsible for the binding of the virus to the membrane of host cells. These spike proteins contain receptor binding domains that recognize the angiotensin-converting enzyme receptor 2 (ACE2), which resides in the lungs, heart, kidneys, and intestines.
The spike protein encompasses two functional subunits. S1, which binds to the host cell receptor, and S2, which mediates the fusion between viral and cellular membranes. Because of the critical role this protein plays in cellular entry, it is a particular focus for gene therapeutics and vaccination targets for COVID-19.
The membrane (M) protein is the most abundant on the viral surface, defining the shape of the viral envelope. This protein has been dubbed the central organizer for COVID assembly, interacting with many other structural proteins. The envelope (E) protein is the smallest of the major structural proteins tasked with several roles. Elucidations built upon the bedrock of SARS and MERS research highlight the (E) protein's job to assemble and release the virus from host cells during viral replication. It is localized on organelles that pertain to intracellular traffickings, such as the rough endoplasmic reticulum and the Golgi apparatus. These M & E proteins play a critical role in turning host cell apparatus into workshops for the virus and for host cells to make new viral particles.
Underneath the capsid (a protein shell that encloses the genetic material of the virus), lies the nuclear capsid or the N protein. This protein is bound to the virus’s single-strand RNA, where all its genetic information is held so that replication could occur. The N protein also inhibits many of the host cell's defense mechanisms and assists the viral mRNA in replicating itself.
Biotechnology: Targeting the Blood Clotting Phenotype of COVID-19
Recent studies show that roughly 30% of high-risk COVID-19 patients succumb to blood clotting which increases the likelihood of strokes and sudden death. Nanotechnology is underway in clinical trials to determine blood clot severity and endothelial cell viscosity, to establish effective blood-thinning medications.
These blood clots occur via virions assembling in plasma membranes after host proteases break down the S1 subunit of the spike protein to the ACE2 receptor. The stress is induced via our own immune system by immune antigens after apoptosis occurs in a given COVID-19 capsid, creating a thrombotic response within our epithelial membranes. Rapid assessment of severe blood clotting could lead to a determination of the correct blood-thinning agent, preventing strokes or other thrombosis-induced jeopardies.
The structure of these S, E, & M proteins found on the surface of these capsids are plasma dependent, resulting in disease-specific coronas. These result in disease-specific biomarkers that can be recognized via mass spectroscopy, allowing for specific disease diagnosis. Nanoparticle-based assays can be conducted, able to adsorb proteins of interest on the surface of these nanoparticles. These assays can be identified via color change, electrical charge, and so on.
Precoating these coronas using fibrinogen, fibrin, or factor VIII and factor XIII tissue plasminogen activator can be done with the biotechnology currently available to us. This could drastically augment the recruitment of analogous proteins in corona. Therefore, precoating these capsids with clot-related proteins such as fibrinogen, fibrin, factor VIII, factor XIII, tissue plasminogen activator, or protein Z could allow for rapid identification of the subtlest signs of clotting in plasma.
Antiviral Medications
Circumventing the COVID-19 vaccines currently on the market, researchers at the National Coordinating Resource Center and other New England Biology firms have developed a series of antiviral medications that have shown some promising results. Nucleotide prodrugs such as Remdesivir, inhibit the viral RNA polymerase chain terminator which can inhibit viral replication altogether. These drugs can be administered to reduce SARS CoV-2- levels in the lungs (excluding the upper respiratory tract), which can ameliorate the COVID-19 disease analog.
One of the leading medications currently being issued to Americans is the administration of convalescent plasma. This convalescent plasma (isolated from recovered patients) works as a passive transporter of antibodies. These antibiotics either bind to the S2 subunit of the spike protein, providing a safeguard to the ACE2 receptor in epithelial cells, or they may coat the virus, tagging them to be engulfed via macrophage cells.
As alternate remedies to the popular vaccines that Pfizer, Moderna, and others are administering, these Nano medications and antiviral treatments can provide an auxiliary defense mechanism to the COVID-19 virus that has so drastically altered our way of living.
References:
- Paige Haas., Monita Muralidharan., Nevan J. Krogan., Robyn M. Kaake., (January 19, 2021). Proteomic Approaches to Study SARS-CoV-2 Biology and COVID-19 Pathology. Journal of Proteome Research, https://pubs.acs.org/doi/10.1021/acs.jproteome.0c00764
- Amir Ata Saei., Shahriar Sharifi., Morteza Mahmoudi., (November 6, 2020). COVID-19: Nanomedicine Uncovers Blood-Clot Mystery. Journal of Proteome Research, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7640964/
- Alvin Powell, Harvard Staff Writer. (December 10, 2020). Fauci says herd immunity possible by fall, ‘normality’ by end of 2021. The Harvard Gazette: Health & Medicine, https://news.harvard.edu/gazette/story/2020/12/anthony-fauci-offers-a-timeline-for-ending-covid-19-pandemic/
- Imke Schröder COVID-19: A Risk Assessment Perspective ACS Chemical Health & Safety (2020) 27 (3), 160-169 DOI: 10.1021/acs.chas.0c00035
Further Reading
Last Updated: May 25, 2021