In multicellular organisms, it is essential to keep a balance between generating new cells and destroying old damaged cells, as well as signaling the immune system when cells are infected.
There are two types of cell death for eukaryotic cells, programmed cell death (apoptosis), and pro-inflammatory cell death (necrosis). The regulation of cell death is complex and failure to properly regulate cell death can result in medical complications.
Image Credit: Kateryna Kon/Shutterstock.com
Types of cell death
Apoptosis is a type of programmed cell death that does not involve the spillage of cellular content into the extracellular matrix and thus, does not trigger an immune reaction. It is characterized by blebbing and shrinking of cells, nuclear and DNA fragmentation as well as global mRNA decay. It an irreversible process and the cell death process is mediated by a group of enzymes - caspases, which can be activated by cell stress or external signals during infection.
In contrast to apoptosis, necrosis involves the spillage of cellular content to the extracellular matrix. This initiates an inflammatory response and recruits immune cells such as neutrophils and macrophages to the spot of cell death. Necrosis is a result of cell injury and is characterized by the loss of membrane integrity. There are two main forms of necrosis: pyroptosis and necroptosis.
Pyroptosis is an inflammatory form of programmed cell death. It is activated upon sensing of a pathogen and involves the assembly of a group of proteins called the inflammasome. The inflammasome activates the enzyme caspase-1 and caspase-1 cleaves the pro-active form of inflammatory cytokines interleukin 1β (IL‐1β) and IL‐18 into their biologically active form, resulting in the release of these cytokines through the membrane pore gasdermin-D, and the activation of the inflammatory response.
Necroptosis is another highly regulated necrosis cell death. It is activated by death receptors such as tumor necrosis factor receptor 1 (TNFR1), necrosis factor‐related apoptosis‐inducing ligand (TRAIL), and Fas receptors. Activation of the above receptors leads to a list of downstream reactions that results in phosphorylation of the mixed lineage kinase domain‐like pseudokinase (MLKL), which then migrates to the cell membrane and permeabilize the cell membrane, resulting in cell death.
Regulation of inflammatory cell death
Inflammatory cell death is tightly regulated by various groups of proteins.
The activation of pyroptosis is regulated by three components: sensor, inflammasome, and caspase-1. Pattern recognition receptors (PRR) senses pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) upon microbe infection. These include Toll-like receptors (TLRs) and NOD-like receptors (NLRs).
Each of these receptors has a specific PAMP or DAMP ligand. Upon binding to their ligands, they initiate the formation of inflammasomes, which then activates caspase-1, triggering pyroptosis.
Cysteine-dependent aspartate-specific proteases (caspases) are a group of proteases that regulates apoptosis and pyroptosis. Caspase-1 is the essential component of the pyroptosis pathway as its activation leads to the release of inflammatory cytokines.
Similarly, necroptosis is also regulated by a sensor and a cascade of reactions leading to permeabilization of the cell membrane. Extrinsic distress signals are sensed by the tumor necrosis factor (TNF) receptor, which then recruits Receptor-interacting serine/threonine-protein kinases (RIPK). RIPK1 and 3 auto-phosphorylate and phosphorylate the downstream MKLK, leading to membrane pore formation and necroptosis cell death.
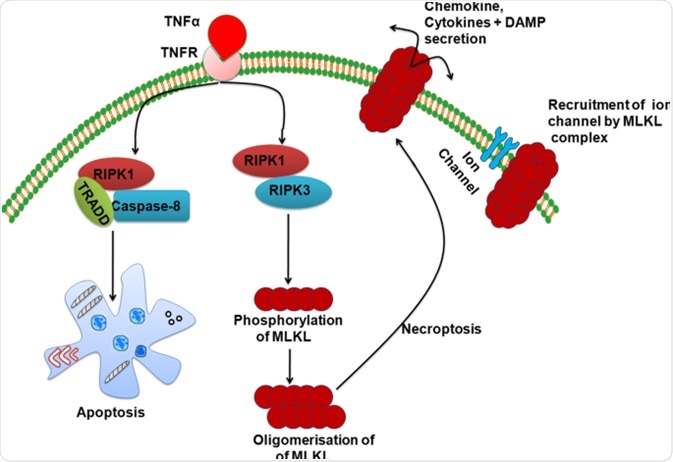
Image Credit: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-018-1235-0
Dysregulation of inflammatory cell death
Dysregulation of inflammatory cell deaths can lead to potentially fatal diseases. For example, the rupture of necrotic cells releases DAMPs, which in turn activate inflammatory cell death in neighboring cells. This creates a positive feedback loop that promotes excessive inflammation.
The presence of AS-associated death domain protein (FADD) or caspase 8 quenches the reaction and halts the positive feedback loop. However, if the negative regulators are faulty, excessive inflammation can lead to organ injury and shock.
Moreover, regulated necroptosis causes neurodegenerative diseases such as Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (MS). Nevertheless, with an increasing understanding of inflammatory cell death regulators, protein inhibitors are developed for effective treatment of diseases.
References:
- D’Arcy, M. S. (2019). Cell death: A review of the major forms of apoptosis, necrosis, and autophagy. Cell Biology International, 43(6), 582-592.
- Dhuriya, Yogesh K, & Sharma, Divakar. (2018). Necroptosis: A regulated inflammatory mode of cell death. Journal of Neuroinflammation, 15(1), 199-9.
- Linkermann, Andreas, Stockwell, Brent R, Krautwald, Stefan, & Anders, Hans-Joachim. (2014). Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nature Reviews. Immunology, 14(11), 759-767.
- Fernández, Daniel Jiménez, & Lamkanfi, Mohamed. (2015). Inflammatory caspases: Key regulators of inflammation and cell death. Biological Chemistry, 396(3), 193-203.
Further Reading
Last Updated: Aug 11, 2022