A breakthrough in breast cancer could enable the development of better approaches to prevent the spread of cancer cells to other organs in the body, thereby effectively bringing down mortality in patients suffering from breast cancer.
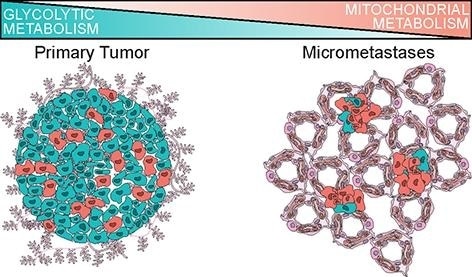
By carrying out scRNAseq in patient-derived models of breast cancer, a UCI-led research team has identified metabolic adaptations that cancer cells acquire as they spread throughout the body. Above is an artistic rendering of the altered metabolism in micrometastatic cells in the lungs compared to primary tumors, opening a new potential avenue to target cancer cell spread in patients. Image Credit: UCI School of Medicine
Based on a study, reported recently in Nature Cell Biology, breast cancer cells tend to change their metabolic strategy in order to metastasize. Mitochondrial metabolism is preferred more by these cells rather than the cycling of sugar (glucose) for energy.
This has important potential clinical implications because it suggests that drugs targeting mitochondrial metabolism may have efficacy for preventing metastatic spread in patients. Historically, tumors were thought to contain dysfunctional mitochondria and be principally sustained by anaerobic glycolysis, or Warburg metabolism. Our work challenges that dogma and shows that breast cancer cells use mitochondrial metabolism during metastatic spread.”
Devon A. Lawson, PhD, Assistant Professor, Department of Physiology and Biophysics, UCI School of Medicine
Lawson is also a member of the Chao Family Comprehensive Cancer Center at the UCI School of Medicine.
There have been major advancements in the detection and treatment of the early stage of breast cancer, but metastasis—spreading of cancer cells in the breast to other organs in the body—causes about 40,000 deaths among women in the United States every year. It is the main cause of all deaths related to breast cancer.
Earlier studies propose that metastasis is triggered by rare primary cancerous cells with special biological properties that allow them to spread. This attribute causes cancer to take root in other locations of the body.
The properties that promote the migration and motility of cells have been well explored. However, the mechanisms that govern the seeding and establishment of small groups of cancerous cells in distal tissues have not as well studied. The reason, in part, is that it is not possible to study metastatic seeding in humans since the detection and analysis of rare cells at the transient stage is difficult in animal models.
Through our research, we established a robust new method for identifying global transcriptomic changes in rare metastatic cells during seeding using single-cell RNA-sequencing and patient-derived xenograft (PDX) models of breast cancer. We found that metastatic cells harbor distinct RNA molecules that are highly predictive of poor survival in patients and alter metabolism in a way that can be targeted therapeutically.”
Ryan Davis, Study First Author and Doctoral Student, Lawson laboratory, UCI School of Medicine
The study was financially supported by a Cancer Center Support Grant, a Center for Complex Biological Systems Support Grant, as well as through support from the National Cancer Institute, National Institutes of Health, the National Science Foundation, the Team Michelle and Friends non-profit organization, the Suzette Kirby Breast Cancer Research Fund, and the V Foundation.
Source:
Journal reference:
Davis, R. T., et al. (2020) Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nature Cell Biology. doi.org/10.1038/s41556-020-0477-0.