Introverts tend to feel encouraged: When cells, similar to certain people, feel things are getting too much, they tend to get into a defense mode, even switching off photosynthesis.
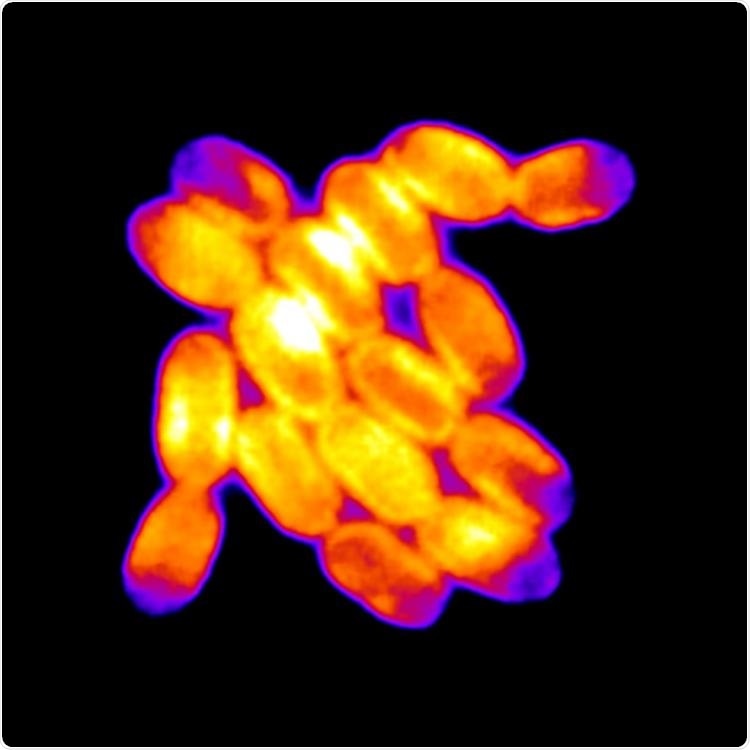
The cells in the middle of this colony of cyanobacteria fluoresce more than those at the edges. Image Credit: Kristin A. Moore and Jeffrey C. Cameron.
In a study published recently, researchers from CU Boulder employed a new microscopic technique to monitor the lives of individual bacteria as they grow and proliferate into complex colonies.
In the process, the team found an unpredicted aspect: whenever these single-celled organisms—a kind of cyanobacteria or blue-green algae—got too squished, they started shutting down the machinery crucial for them to convert sunlight into sugar.
Jeffrey Cameron stated that the small organisms reduced their growth rate to a greater extent. Cameron is an assistant professor in the Department of Biochemistry and also co-author of the new study.
If a cell is between a rock and a hard place, internally everything says, ‘Yes, I have nutrients. I want to grow. But there also has to be a feedback that says, ‘I need to turn off photosynthesis so I don’t expand and rupture.”
Jeffrey Cameron, Study Co-Author and Assistant Professor, Department of Biochemistry, University of Colorado Boulder
The study outcomes appear in the Nature Microbiology journal and offer new insights into this process that helps sustain most life on Earth—and specifically how organisms control photosynthesis when the process becomes difficult.
Cameron and his team’s outcomes also help researchers to develop tailor-made microbes that can eventually assist in the conversion of light into electricity or even develop living buildings.
Bacterial pancakes
Cameron explained that it all started almost by accident. He started this study several years ago keeping a simple goal in mind—along with his team, he intended to monitor the behavior of individual cells within a bacterial colony through their whole life cycle.
The task was difficult because simple-looking organisms like cyanobacteria can form quite complex structures.
The cells on the outside of a colony are exposed to a lot of light, while those on the inside have low light exposure. Over time as they grow and accumulate more cells, they shade themselves.”
Jeffrey Cameron, Study Co-Author and Assistant Professor, Department of Biochemistry, University of Colorado Boulder
Cameron developed a technique for culturing cyanobacteria, such that they spread out similar to flat pancakes, observing every cell in a growing colony. He observed something strange when he started looking at these two-dimensional growths under a microscope—the more the colonies expanded and the more bacteria inside started to squeeze together, the more they began to glow, or fluoresce, under a specific light source.
Cameron explained that the microbes were emitting heat out to their environment, similar to a person sweating on a tightly packed city bus.
Cameron said, “I dropped everything and spent the next four years figuring out what was happening.”
He and his team found that not all the cells in a colony glow the same. The cyanobacteria within a colony, for instance, fluoresced to a greater extent than those on the fringes. They also grew very gradually, proliferating in two at nearly half the rate as those exterior to the colony. In other words, when the cells get smushed, they tend to fluoresce.
Cameron added, “When the cells become confined, and they can’t expand, they become highly fluorescent.”
Tiny antennae
There arose a question on why the interior cells were sweating so much. The answer is that one has to have profound knowledge about the phycobilisome. This small, protein-based structure acts as the antenna of the cell.
Several phycobilisomes reside within cyanobacteria, capturing sunlight and transporting it to the reaction sites where the energy gets converted into glucose or sugar.
Cameron and his team identified that when the cyanobacteria became highly confined, they started to lose their phycobilisomes.
Cameron stated, “If the cyanobacteria got more light than they could use for photosynthesis, these antennae would literally pop off.”
The microbes could not use the entire energy; thus, to prevent glutting, they switched off the process of photosynthesis.
The study outcomes demonstrate the functioning of dynamic single-celled organisms—they have an enormous set of tools for staying healthy under a dynamic social environment.
The study shows that the social environment could someday assist researchers to put photosynthesis to work by tapping sunlight to engineer more sustainable buildings or to create other biology-based tools.
We might be able to develop small-scale machines that are using light to perform computations or work.”
Jeffrey Cameron, Study Co-Author and Assistant Professor, Department of Biochemistry, University of Colorado Boulder
It is a completely new approach to think about trapping some rays.
Source:
Journal reference:
Moore, K. A., et al. (2020) Mechanical regulation of photosynthesis in cyanobacteria. Nature Microbiology. doi.org/10.1038/s41564-020-0684-2.