According to a new study performed by the University of Wisconsin-Madison, deleting a gene from insulin-producing cells prevents the development of Type 1 diabetes in mice, by sparing the cells from being attacked by their own immune system.
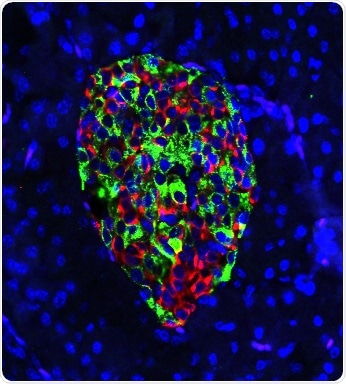
Cells in the pancreas of gene-edited mouse produce nearly equal amounts of the hormones insulin (green) and glucagon (red). In mice that develop Type 1 diabetes, green would predominate, and insulin production would draw the deadly attention of a disordered immune system. Image Credit: Hugo Lee.
This cellular sleight-of-hand may indicate ways to prevent Type 1 diabetes in individuals who have a higher risk of developing this condition, and also other disorders where the immune system targets the own cells of the body.
People suffering from Type 1 diabetes—once known as juvenile diabetes—produce little or no insulin, a hormone that is required to make energy from their blood sugar.
During the early stage of the disease, the frontline soldiers of the patients’ immune system, known as T cells, wrongly detect the insulin-producing beta cells as a threat and destroy them, resulting in a complete deficiency of insulin.
The chaos that results from this mechanism should be controlled for the rest of the patient’s life with insulin shots, diet, and blood sugar measurement.
Globally, as many as 20 million persons are suffering from Type 1 diabetes, which contributes to stroke, high blood pressure, nerve damage, and glaucoma. In the United States, Type 1 diabetes shortens an individual’s life span by more than 10 years.
The thing is, individuals who are at high risk can be identified. They have autoantibodies in their blood serum, meaning we can actually tell who is going to develop Type 1 diabetes within a couple of years. But there’s not much for clinicians to do but send them home, because there’s no cure for Type 1 diabetes.”
Feyza Engin, Study Lead Author and Biomolecular Chemistry Professor, University of Wisconsin–Madison
The study was recently published in the Cell Metabolism journal.
Engin’s laboratory modified a line of mice that were genetically ordained to develop Type 1 diabetes. Just before the immune attack typically begins, the researchers removed a gene, known as IRE1-alpha, from the beta cells; this gene plays a role in the mouse cells’ reaction to stress.
Engin believed that the removal of the IRE1-alpha gene from insulin-producing beta cells would result in accelerated diabetes. However, the removal of this gene made a surprising and remarkable difference in the mice.
“We expected the beta cells would die soon,” added Engin. “Instead, my students told me that the blood glucose levels of the mice were becoming normal following an initial increase lasting a couple of weeks. I couldn’t believe it. I said, ‘What? No. Just measure it one more time.’”
The beta cells were, in fact, turning out to be normal insulin producers. But before that, the cells were taking a step backward into immaturity.
“Once we remove this gene, it’s almost like the beta cells are undergoing a disguise,” added Engin, who was joined by Hugo Lee, the study’s first author, and a graduate student, in publishing the study results. “They lose their mature identity. They de-differentiate and exhibit features of progenitor cells, and express hormones of other cell types in addition to insulin.”
If this de-differentiation occurs before an auto-immune response places the beta cells in danger, then the T cells encountered by them would react in a different way.
When they de-differentiate, they don’t act like typical beta cells anymore. They reduce the expression of many genes that signal to immune cells, ‘Come and eat me!’. Those signals go down, and that actually is altering the diabetogenic activity of T cells. They don’t really recognize the beta cells as a problem anymore. They don’t attack.”
Feyza Engin, Study Lead Author and Biomolecular Chemistry Professor, University of Wisconsin–Madison
And later, just as significantly, the de-differentiated, immature beta cells again differentiate into mature, functional beta cells.
“The mice experienced a little transient hyperglycemia. They have relatively high blood sugar, which isn’t dangerous, for a few weeks,” added Engin, whose laboratory is supported by the Juvenile Diabetes Research Foundation and the National Institutes of Health. “But then the beta cells get back to work, and make insulin-like they’re supposed to.”
The T cells modify their activity and maintain the change, and leave the beta cells alone as long as the laboratory has tracked the mice to date.
That’s the beauty of it. Even after the beta cells come back, the T cells leave them alone. They still have no diabetogenic activity one year later, which is like 40 or 50 years in a human life.”
Feyza Engin, Study Lead Author and Biomolecular Chemistry Professor, University of Wisconsin–Madison
A pair of drugs are currently being tested in clinical trials for Type 1 diabetes. These drugs target the beta cells’ stress response—including a drug whose efficacy Engin had identified in mice while she was working at Harvard University.
The new findings made by Engin’s laboratory may help guide the way candidate diabetes drugs are utilized in clinical trials or help develop novel treatments. And they could have an analogous effect in other auto-immune diseases—such as multiple sclerosis, lupus, and arthritis—where the activity of a specific cell type draws dysfunctional immune attention.
“We’ve found a very important time point where de-differentiation helps greatly reduce the immune cells’ diabetogenic activity. If you can determine an appropriate cell targeted by auto-immune response, and make those victim cells less functional, less mature in the beginning, maybe they can avoid their role in the progress of other diseases, too,” Engin concluded.