Human stem cells have been typically perceived as one of the potential cell sources for cardiac regeneration treatment.
However, the substandard performance following transplantation into the failing hearts has limited their applications in clinical use.
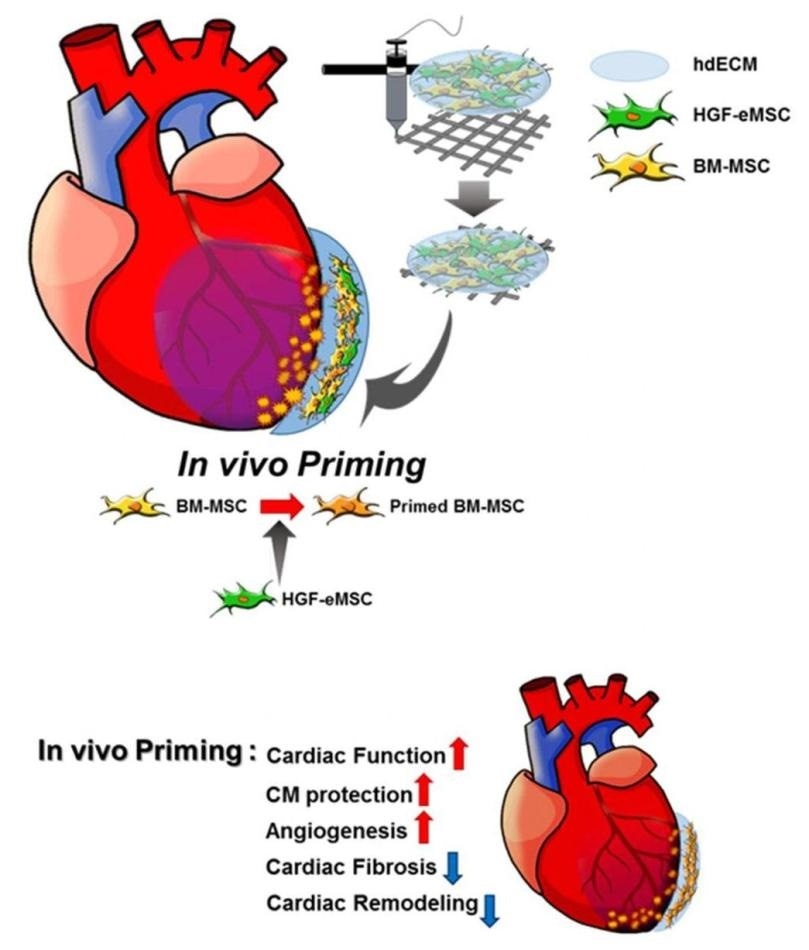
Schematic diagram of the underlying mechanism of in vivo priming. The cardiac patch is 3D-printed with a bioink produced by the mixture of porcine heart-derived extracellular matrix and the MSCs. The patch is then implanted on the infarct area of the rat heart. Image Credit: © City University of Hong Kong/Science Advances.
A stem cell biologist from City University of Hong Kong (CityU), along with his colleagues, has recently developed a new method, known as in vivo priming, which could help resolve this problem. This strategy could allow researchers to “train” the stem cells to remain strong even after they are implanted into the damaged heart through the 3D-printed bandage-like patch.
The positive outcomes of the research demonstrated that an in vivo priming method can be an effective way to improve cardiac repair.
Dr. Ban Kiwon, Assistant Professor of CityU’s Department of Biomedical Sciences, achieved this latest breakthrough by working with experts and cardiologists in 3D printing from South Korea.
The results of the study titled “In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes the therapeutic potential for cardiac repair” were published in the new issue of the scientific journal, Science Advances.
Harsh environment in failing hearts hinders stem cell survival
One of the recommended methods for treating myocardial infarction—often called heart attack—with regeneration therapy is to directly administer the human stem cells into the failing hearts.
Specifically, human mesenchymal stem cells (hMSCs) have been regarded as a competitive agent for clinical applications based on their established safety and major paracrine effects that support the formation of new blood vessels and inhibition of cell death.
But “the clinical trial results are disappointing as the micro-environment of a failing heart is very harsh for the injected hMSCs to stay alive,” stated Dr Ban.
Traditionally, priming is carried out in vitro (that is, outside a living organism) before transplanting the cells into the heart.
But the effects of priming done in this way usually last for two or three days only. To extend the duration of the priming effect, I have come up with an idea of ‘in vivo priming’, which means the hMSCs are primed directly on the failing hearts.”
Dr Ban Kiwon, Assistant Professor, Department of Biomedical Sciences, City University of Hong Kong
Novel strategy: in vivo priming of hMSCs
To demonstrate this concept, the scientists placed two kinds of MSCs into a customized 3D-printed patch, that is, the genetically engineered MSCs that have human hepatocyte growth factor protein, and the human bone marrow-derived MSCs.
Hepatocyte growth factor (HGF) has been implicated in numerous biological activities, like anti-fibrotic activities, the formation of blood vessels, and cell survival, while it is also crucial in wound healing and regeneration of adult organs.
Similar to a bandage, the patch was subsequently implanted to the surface of rats’ hearts—that is, over the infarct region of the myocardial-infarction-induced heart.
“The genetically engineered MSCs can continuously secret human HGF protein to prime the hMSCs within the patch and make them ‘stronger’,” added Dr. Ban.
Dr. Ban further informed that rather than directly administering the genetically engineered cells into the heart, enclosing the cells in the patch for placing on top of the heart can help inhibit the adverse outcomes, including mutation.
The patch is developed by 3D printing of the extracellular matrix hydrogel extracted from a pig’s heart and activating the micro-environment specific to cardiac tissues.
The researchers observed a higher survival rate in the primed hMSCs than that of the unprimed ones in the patches, implanted in the failing hearts.
The empowered hMSCs discharged higher amounts of paracrine factors that are useful for regenerating vasculatures and repairing damaged cardiac muscle tissues.
We found that the primed cells can survive even after 8 weeks in the patch after implantation to the heart. Also, there is a significant improvement in cardiac function as well as vessel regeneration comparing to the unprimed cells.”
Dr Ban Kiwon, Assistant Professor, Department of Biomedical Sciences, City University of Hong Kong
Great improvement of the priming effect
Our team is the very first to achieve priming in hearts in vivo. But more importantly, by showing that in vivo priming of hMSCs can enhance the therapeutic potential for cardiac repair, we hope our study can bring significant implications for related stem cell therapy in future.”
Dr Ban Kiwon, Assistant Professor, Department of Biomedical Sciences, City University of Hong Kong
The researchers took more than two years to arrive at these amazing results. They will now analyze the potential of performing the experiments on larger animals and even clinical trials, and also investigate the possibility of altering the patch’s structure.
Source:
Journal reference:
Park, B.-W., et al. (2020) In vivo priming of human mesenchymal stem cells with hepatocyte growth factor–engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Science Advances. doi.org/10.1126/sciadv.aay6994.