According to a researcher from Hokkaido University and collaborators from Japan-based Sapporo Medical University, exercise can boost strength in muscle-degenerating disorders only when aging occurs in a certain type of muscle cell.
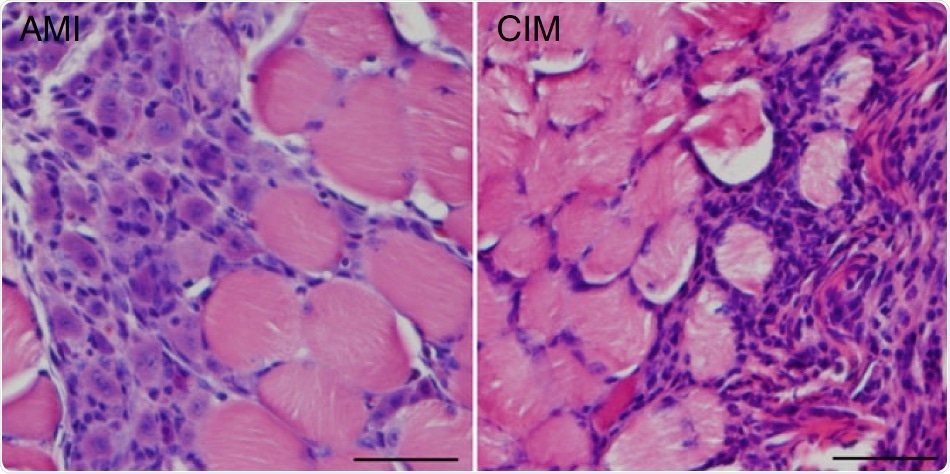
Regenerating fibers with central nuclei in muscles suffering from acute muscle injury (AMI; left), and fibrosis and inflammatory cells in chronic inflammatory myopathy (CIM; right). Image Credit: Yuki Saito et al., Nature Communications. February 14th, 2020.
The researchers’ findings using mice models were published in the Nature Communications journal.
Idiopathic inflammatory myopathies can be defined as rare medical disorders that can cause inflammation, fibrosis, and muscle weakness. Apart from medications, exercise can be an intense therapy for a certain group of patients.
However, in cases of chronic inflammatory myopathy, exercise can in fact induce fibrosis and inflammation in muscles. Researchers have been trying to figure out why exercise benefits certain myopathies but does not for others.
Takako S. Chikenji from Hokkaido University teamed up with Yuki Saito and Mineko Fujimiya from Japan-based Sapporo Medical University to analyze why a specific type of muscle cell, known as fibro-adipogenic progenitors (FAPs), reacts differently to physical exercise based on the type of myopathy. Such cells are the major regulators of the muscle stem cells that are required for regeneration.
The researchers then observed the mice suffering from this disorder and analyzed what exactly occurs when these animals exercise. In mice with an acute muscle injury that replicates idiopathic inflammatory myopathy, the team discovered that FAPs initially increased but after seven days, they subsequently returned to pre-damage levels. The FAPs ultimately died and were ingested by phagocytes, which are cell-eating immune cells.
FAPs continued to proliferate in mice with chronic inflammatory myopathy for a period of 14 days and were impervious to cell death and clearance by the immune cells.
The researchers further investigated and discovered that the FAPs in mice with acute muscle injury displayed intense signs of aging following exercise but the same was not displayed by FAPs in mice having chronic inflammatory myopathy.
The scientists also discovered that while normal FAPs encouraged regeneration of muscle cells following acute muscle injury, FAPs that lacked a cellular aging-inducing factor did not do so. These outcomes collectively revealed that the aging of FAPs after exercise is essential to establish a state of regenerative inflammation that leads to the regeneration of muscle cells
Additionally, exercise combined with administration of drugs that promote cellular aging effectively restored FAP aging, and enhanced function and regeneration of muscles in mice exhibiting chronic inflammatory myopathy.
Our findings demonstrate that exercise leads to muscle degeneration in chronic inflammatory myopathy because FAPs accumulate when they fail to age. Pharmacological induction of FAP senescence dramatically improved the therapeutic effects of exercise in mice with chronic myopathy. Further research is needed to investigate whether this strategy could be used to treat this condition in humans.”
Takako S. Chikenji, Researcher, Hokkaido University
Source:
Journal reference:
Saito, Y., et al. (2020) Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nature Communications. doi.org/10.1038/s41467-020-14734-x.