Every picture tells a story... none more so than this detailed visualization of a strain of the norovirus.
Created from 13,000 separate images taken by an electron microscope, it reveals in rich detail the structure of the virus. It shows bump-like protrusions on the outside of the virus capsid, the protein shell that holds the genome of the virus.
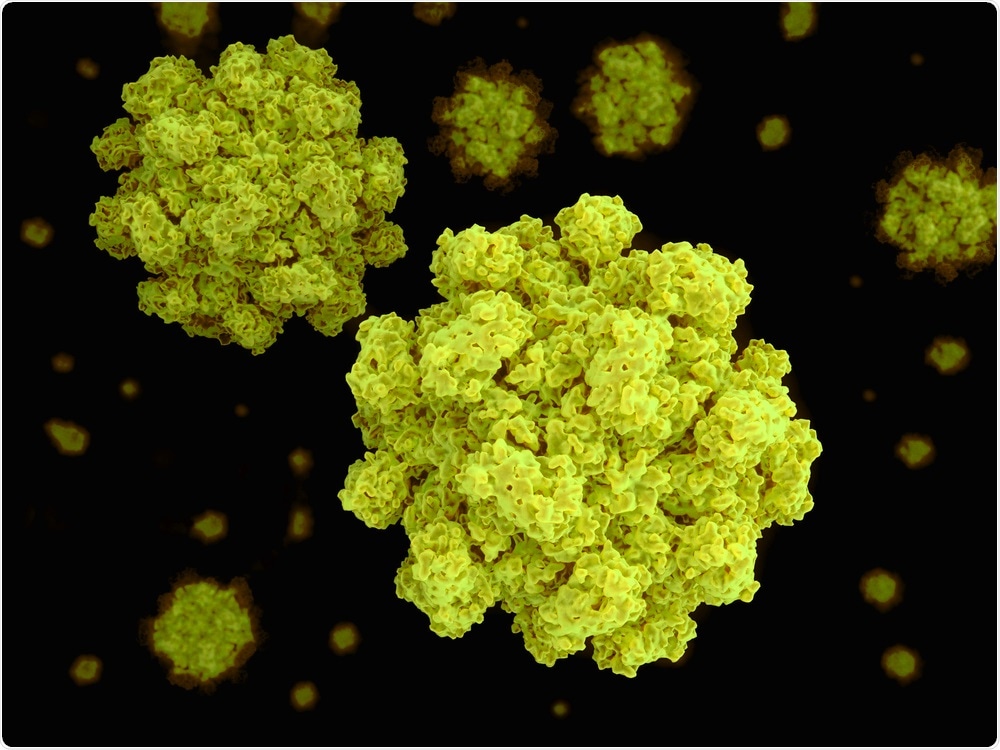
Image Credit: Juan Gaertner/Shutterstock.com
Joseph Snowden, a Ph.D. researcher from the Astbury Centre for Structural Molecular Biology at the University of Leeds, said it revealed that the protein casing did not keep a fixed shape - as the protrusions on the surface would extend, retract and rotate.
This dynamic shape-shifting may hold clues as to why the noroviruses are such potent pathogens, responsible for over 200,000 deaths worldwide each year, mainly in low-to-middle-income countries. In the UK, noroviruses cause the winter vomiting bug that forces the closure of schools and hospitals.
To identify subtle changes to the structure of these protein shells, the scientists processed the data from the images with a supercomputer, using a method called "focussed classification".
For safety reasons, the researchers used a mouse norovirus in their study, which closely resembles the human noroviruses.
Mr. Snowden said: "The constant changing or morphing of the virus shell may enable it to confuse the body's defense systems.
"Immune systems work on the basis of molecular shape. The body's defenses will produce proteins that bind to pathogens to prevent them from infecting host cells. But if the shape of the virus is constantly changing, the body's defense systems may be unable to act efficiently."
The protrusions seem able to move independently or in a coordinated fashion and the scientists believe this may enable the virus to prime itself ready for infection depending on where it is - i.e. if it is in the digestive tract of a host organism.
It is believed that this study is the first study to use the focussed classification computing tool to investigate the structure of a norovirus.
Dr. Morgan Herod, one of the senior authors on this study, also from the University of Leeds, hopes that a greater understanding of the structure of the norovirus may help with vaccine development, which so far has been unsuccessful.
The University of Leeds has a strong track record in the use of virus-like particles (VLPs) to create vaccine candidates, for example against polio. VLPs are harmless proteins modeled on the structure of a virus's casing and they fool the immune system into thinking the body is under attack, prompting an immune response. "
Dr. Herod
"Our research shows that the capsid or protein shell of norovirus is dynamic - and perhaps we have to look at VLPs that are better able to mimic this aspect of norovirus structure."
"That will make the vaccine development task a little harder."