The structure of a protein that plays a role in importing vitamin B12 into the cells of tuberculosis (TB)-causing bacterium has been revealed by scientists from the Netherlands, Russia, the United States, and Sweden.
The study results were published in the Nature journal.
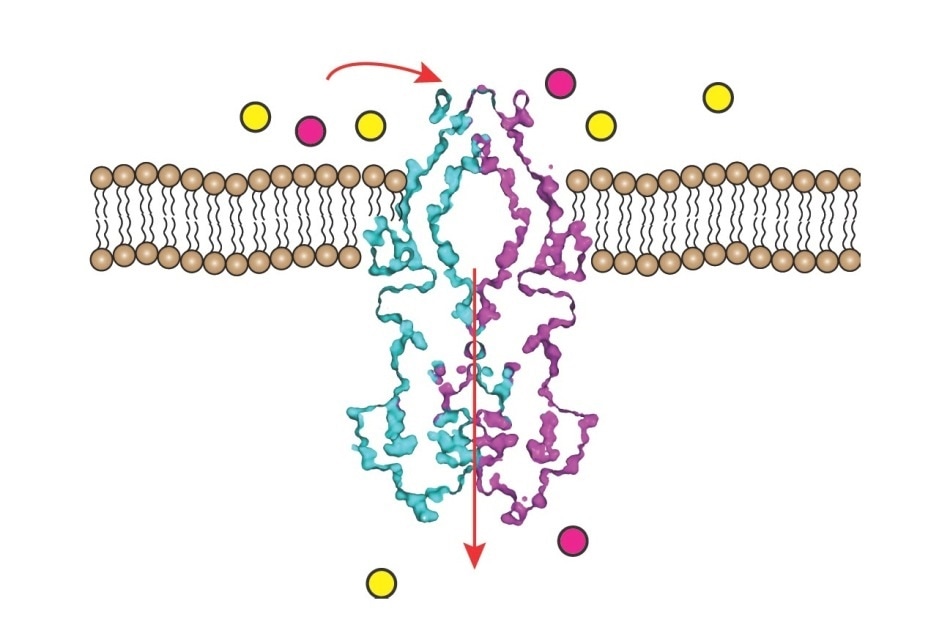
Rv1819c protein transports a positively charged substrate, shown in pink, across the membrane into the cell. Image Credit: Albert Guskov/Moscow Institute of Physics and Technology.
Mycobacterium tuberculosis is known to cause TB. This bacterium essentially requires vitamin B12 for its proliferation, which is also termed cobalamin.
While vitamin B12 can be produced internally, it is relatively easier for the bacteria to import this vitamin from the environment, and the consumption of cobalamin by these pathogens is directly connected with the progression of TB.
But except for three cobalamin-associated proteins, no known importer of vitamin B12 has been identified in the genome of the pathogen.
Although one of the proteins, called Rv1819c, was considered a candidate for the transport of vitamin B12, the amino acid sequence analysis proposed that it probably works in the other way—that is, as an exporter.
This contradiction was addressed by the authors of the new study published in the Nature journal. The researchers discovered that the protein certainly has the ability to import vitamin B12 and employed single-particle cryo-electron microscopy to describe how it functions.
The researchers performed an experiment in which they used a cell line of Escherichia coli and manipulated it such that it lacked any B12 transporters.
When the bacteria were placed in a cobalamin-rich culture medium, they did not grow until the scientists injected them with the Rv1819c protein from Mycobacterium tuberculosis, which caused them to proliferate.
A mutated cell line was also used by the researchers to determine that the transport mediated by the Rv1819c protein relies on ATP hydrolysis.
The protein’s three-dimensional (3D) structure was resolved by the researchers. The structure contains two irregularly sized interconnected chambers, with the smaller one projecting on the inside of the cell membrane and the larger one situated in the same cell membrane. The region between both the chambers is narrow and restricted by a loop of 17 amino acids.
The large cavity has a capacity of around 7.7 nm3, which is sufficient to house six or seven vitamin B12 molecules.
The outside of the chamber is sealed with a cap, maybe opening instantaneously to trap molecules from the outside environment. The cavity has an affinity to hydrophilic molecules as it is inlaid with polar and negatively charged amino acids.
This mechanism shows why the Rv1819c transporter, in spite of not being highly selective, prefers positively charged and polar substrates. For instance, the researchers demonstrated that apart from vitamin B12, the Rv1819c protein is capable of transporting a cancer drug known as bleomycin.
But at the same time, this protein did not work with biotin, perhaps due to the presence of negatively charged groups in the latter molecule.
The portion of the protein within the cell is closed with a bi-valved gate, which interacts with the sites where the cell breaks down ATP molecules to produce energy. Two of these molecules have to be consumed to open the gate, demonstrating why Rv1819c-mediated transport depends on ATP hydrolysis.
Our study takes us one step further in understanding how tuberculosis develops. We now know how the pathogen acquires vitamin B12, which is vital for it. Importantly, the mechanism we humans use to import cobalamin is very different, making Rv1819c an excellent target for developing anti-TB drugs.”
Albert Guskov, Study Co-Author and Head, Moscow Institute of Physics and Technology
Guskov continued, “The discovery of this unexpected function of the protein that has been regarded as an exporter rather than an importer offers insights into the possible mechanisms of antibiotic delivery into pathogen cells. Aminoglycosides, for example, are positively charged, so they cannot freely penetrate the membrane. Previously, we only knew these drugs somehow got into the cell. We can now say that Rv1819c and similar proteins might well be the transporters involved.”
The study involved scientists from SLAC National Accelerator Laboratory, the University of Groningen in the Netherlands, Stockholm University in Sweden, and Stanford University in the United States.
Source:
Journal reference:
Rempel, S., et al. (2020) A mycobacterial ABC transporter mediates the uptake of hydrophilic compounds. Nature. doi.org/10.1038/s41586-020-2072-8.