With the number of COVID-19 cases reducing in the United States and elsewhere, public health officials are attempting to understand the proportion of people who have been infected by the coronavirus.
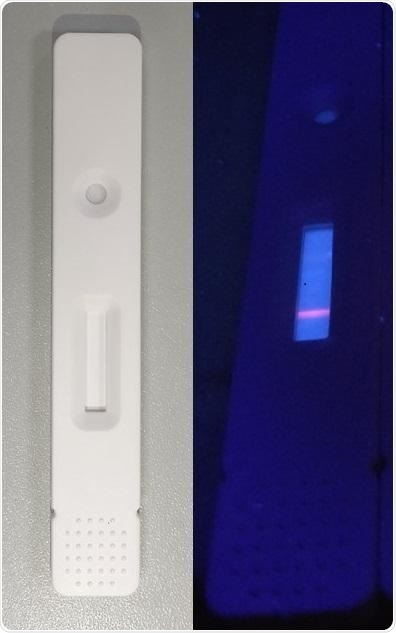
A new lateral flow immunoassay can detect antibodies against SARS-CoV-2, which appear as a bright orange line when placed on a fluorescence reader (right). Image Credit: Guanfeng Lin.
Recently, a proof-of-concept study, published in ACS’ Analytical Chemistry, explains a rapid, sensitive test that detects antibodies against the SARS-CoV-2 (coronavirus) in human blood.
The latest test could help physicians to monitor an individual’s exposure to the disease and also verify the suspected COVID-19 cases that tested negative by other techniques.
Since symptoms of the COVID-19 infection range from mild to severe, with a few people evidently showing no symptoms, the number of people infected with the coronavirus at some point in time could be relatively higher than the number of the confirmed COVID-19 cases.
With the U.S. states set to reduce lockdown restrictions, extensive testing of the general population will be crucial to detect people right at the initial stages of disease, or persons who do not show any symptoms but can still infect other individuals.
Moreover, while more studies have to be performed, people who have antibodies to the virus could be resistant to imminent COVID-19 outbreaks. Hence, to help detect individuals with past or present exposure to coronavirus, Lei Yu, Yingsong Wu, Guanfeng Lin, and their collaborators wanted to devise a rapid and sensitive antibody test.
The scientists based their test on a method known as a lateral flow immunoassay (LFA); an example of this type of assay is the home pregnancy test. The researchers linked a viral coat protein to a particular region on a strip of nitrocellulose and subsequently added the human serum.
This serum traveled from one end of the strip to the other, and also to any antibodies against the viral protein adhered to that area on the strip.
Then using a fluorescently labeled antibody, the researchers identified the anti-SARS-CoV-2 antibodies. This fluorescence-based detection is relatively more sensitive compared to some other LFAs, like the pregnancy tests, that can be read even by the naked eye.
Next, the scientists analyzed the new assay on seven more serum samples collected from patients infected with COVID-19, and 12 samples obtained from persons who had tested negative for the same disease using reverse transcriptase-polymerase chain reaction (RT-PCR).
RT-PCR is a standard diagnostic test that intermittently fails to identify positive cases. The latest assay accurately diagnosed all the seven serum samples as positive, and also another “negative” case that had suspicious clinical symptoms, in just 10 minutes for each sample.
This immunoassay could be used for analyzing past exposures, tracking a patient’s recovery, confirming negative diagnoses, and detecting the recovered persons with increased levels of antibodies as potential convalescent plasma donors, the scientists concluded.