An interdisciplinary research team at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at UCLA Health has created the world’s first roadmap of the development mechanism of human skeletal muscles, including the development of muscle stem cells.
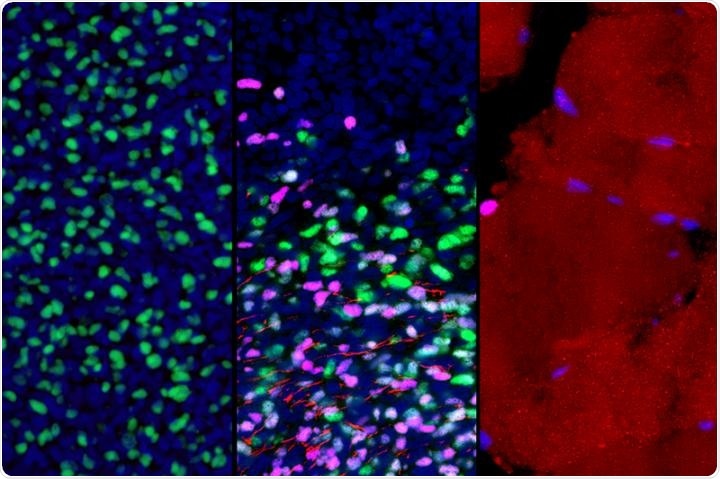
These microscopic images show gene expression in muscle stem and progenitor cells as they mature from early development to adulthood (left to right). As part of this process, the cells switch from actively expressing one key gene (green) to another (violet); this is accompanied by the growth of muscle fibers (red). Image Credit: Broad Stem Cell Research Center.
In a study published in the peer-reviewed journal Cell Stem Cell, the researchers discovered different types of cells found in the tissues of skeletal muscles, right from the initial embryonic development through to adulthood.
The researchers initially targeted the muscle progenitor cells, which play a role in the formation of muscles before birth, and muscle stem cells, which play a role in the formation of muscles after birth and in the regeneration from injury throughout life.
Then, based on this, they mapped out how the cells’ gene networks, the genes of which are both active and inactive, change as the cells develop.
The roadmap is crucial for the scientists, who are aiming to create muscle stem cells in the laboratory for use in regenerative cell therapies meant for serious muscle diseases such as sarcopenia, the age-related loss of muscle mass and strength, and muscular dystrophies.
Muscle loss due to aging or disease is often the result of dysfunctional muscle stem cells. This map identifies the precise gene networks present in muscle progenitor and stem cells across development, which is essential to developing methods to generate these cells in a dish to treat muscle disorders.”
April Pyle, Study Senior Author, UCLA Health
Pyle is also a member of the Broad Stem Cell Research Center.
Scientists in Pyle’s laboratory and other scientists across the world are capable of producing skeletal muscle cells from human pluripotent stem cells, cells that can renew on their own and develop into any type of cell in the body.
But to date, no appropriate way was available to determine where these cells fall on the gamut of human development.
We knew that the muscle cells we were making in the lab were not as functional as the fully matured muscle stem cells found in humans. So we set out to generate this map as a reference that our lab and others can use to compare the genetic signatures of the cells we are creating to those of real human skeletal muscle tissue.”
Haibin Xi, Study First Author, UCLA Health
Xi works as an assistant project scientist in Pyle’s laboratory.
The researchers created this resource by collecting highly specific data of two different groups of skeletal muscle cells, one group from the human body, spanning from the fifth week of embryonic development to middle age, and the other group extracted from human pluripotent stem cells that were produced in the laboratory. Then, the genetic signatures of the cells from both sources were compared by the researchers.
The researchers acquired 21 samples of human skeletal muscle tissues from their UCLA colleagues and also from collaborators at the University of Tübingen in Germany and the University of Southern California.
With regard to the pluripotent stem cell-derived muscle cells, the researchers assessed the cells that were developed using their own special technique as well as the techniques of many other teams.
Pyle’s lab teamed up with the lab of Kathrin Plath, a UCLA professor of biological chemistry and a member of the Broad Stem Cell Research Center, to perform high-throughput droplet-based single-cell RNA sequencing of all the samples.
This new technology allows scientists to detect the gene networks that exist in a single cell and is capable of processing scores of cells simultaneously.
Exploiting the Plath lab’s bioinformatics expertise and the power of this novel technology, the researchers detected the genetic signatures of different types of cells from human pluripotent stem cells and tissues.
Subsequently, the researchers developed computational techniques to target stem cells and muscle progenitors and mapped out their gene networks related to each developmental stage.
This allowed the scientists to match the genetic signatures present in the pluripotent stem cell-derived muscle cells with their matching locations on the map of human muscle development.
The team found that pluripotent stem cell-derived muscle cells generated by all the tried and tested methods looked like muscle progenitor cells at an initial developmental state and did not align with adult muscle stem cells.
Apart from underscoring the real maturity of the laboratory-produced cells, this study also yielded details about other types of cells located in skeletal muscle tissue across development, as well as in populations obtained from human pluripotent stem cells.
All these cells play a vital role in the maturation of muscle cells and could be critical to enhancing the methods to produce and support muscle stem cells grown in a dish.
We found that some methods to generate muscle cells in a dish also produce unique cell types that likely support the muscle cell. And so now our questions are, what are these cells doing? Could they be the key to producing and supporting mature and functional muscle stem cells in a dish?”
April Pyle, Study Senior Author, UCLA Health
Going forward, Pyle and her collaborators will work on harnessing another resource to develop more improved techniques for producing muscle stem cells from human pluripotent stem cells in the laboratory.
According to Pyle, by focusing on the stem cell-related gene expression networks and the identified supportive cell types, they can create high-powered muscle stem cells that can prove handy for regenerative therapies in the days to come.
Source:
Journal reference:
Xi, H., et al. (2020) A Human Skeletal Muscle Atlas Identifies the Trajectories of Stem and Progenitor Cells across Development and from Human Pluripotent Stem Cells. Cell Stem Cell. doi.org/10.1016/j.stem.2020.04.017.