For the first time, a research team led by Martin Fussenegger, a professor at ETH Zurich, has successfully used electric current to directly manipulate gene expression.
The study offers the basis for medical implants that can be turned on and off by using electronic devices placed outside the body.
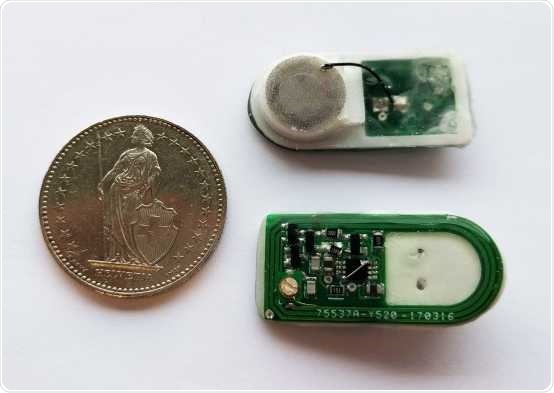
Prototype of the implant: A cable connects the round cell-filled chamber with the control electronics (green). The whole construction is about the size of a two Swiss francs coin. Image Credit: Krzysztof Krawczyk/ETH Zurich.
The latest approach works like this. A device containing an electronic control unit and insulin-producing cells is embedded into the body of diabetic patients.
When the patients consume something and their glucose level rises immediately, they can simply use an app installed on their mobile phone to activate an electrical signal, or they can preconfigure the app to do this automatically if the meal has been entered beforehand.
After a short period of time, the cells release the required amount of insulin needed to control the blood sugar level of the patient.
While this may look like science fiction, it may turn out to be a reality in the days to come. A group of scientists, headed by Martin Fussenegger—ETH Professor of Biotechnology and Bioengineering at the Department of Biosystems Science and Engineering in Basel—has demonstrated its prototype for such an implant in the latest article published in the Science journal.
This is the first study to analyze how the expression of genes can be directly triggered and controlled using electrical signals. When testing their new method in mice, the scientists established that it functioned perfectly.
The Basel-based researchers have enormous experience in designing genetic networks and implants that react to the body’s particular physiological states, like blood sugar levels that are too low or blood lipid levels that are too high.
Even though such networks react to biochemical stimuli, they can also be regulated by alternative, external factors like light.
We’ve wanted to directly control gene expression using electricity for a long time; now we’ve finally succeeded.”
Martin Fussenegger, Professor of Biotechnology and Bioengineering, Department of Biosystems Science and Engineering, ETH Zurich
A circuit board and cell container hold the key
The implant designed by the scientists comprises several parts. On one side, it features a printed circuit board (PCB) that houses the control electronics and receiver, and on the other, it has a capsule consisting of human cells. A small cable connects the PCB to the cell container.
A radio signal from outside the body triggers the electronics in the implant, which, in turn, sends the electrical signals directly to the cells. The electrical signals excite a unique combination of potassium and calcium channels. This, in turn, activates a signaling cascade in the cell that regulates the insulin gene.
The cellular machinery then loads the insulin into vesicles caused by the electrical signals to merge with the cell membrane, producing the insulin within a matter of minutes.
Coming soon: the internet of the body
For Fussenegger, this latest advancement offers several benefits. He explained, “Our implant could be connected to the cyber universe.”
Patients or doctors could use an app to directly intervene and activate the production of insulin, something they could also perform remotely across the internet once the implant has conveyed the required physiological data.
A device of this kind would enable people to be fully integrated into the digital world and become part of the Internet of Things—or even the Internet of the Body.”
Martin Fussenegger, Professor of Biotechnology and Bioengineering, Department of Biosystems Science and Engineering, ETH Zurich
Fussenegger takes a level-headed view whenever there is a potential risk of attacks by hackers.
People already wear pacemakers that are theoretically vulnerable to cyberattacks, but these devices have sufficient protection. That’s something we would have to incorporate in our implants too.”
Martin Fussenegger, Professor of Biotechnology and Bioengineering, Department of Biosystems Science and Engineering, ETH Zurich
As such, the greatest challenge for Fussenegger is the genetic side of things. To make sure that no damage is caused either to the genes and cells, he and his research team have to do more research into the highest current that can be used. They must also improve the link between the cells and the electronics.
The last obstacle to tackle is to find a new, easier, and simpler way to substitute the cells employed in the implant—something that should be done at least every three weeks. For their experiments, Fussenegger and his research team fixed two filler necks to their model to substitute the cells to find a more viable solution.
But before the researchers’ system can be used in humans, it must pass through a whole gamut of clinical tests.
Source:
Journal reference:
Krawczyk, K., et al. (2020) Electrogenetic cellular insulin release for real-time glycemic control in type 1 diabetic mice. Science. doi.org/10.1126/science.aau7187.