During the development of embryos, tissues flow and reorganize significantly on timescales within minutes. This reorganization involves epithelial tissues that conceal inner linings and outer surfaces of blood vessels and organs.
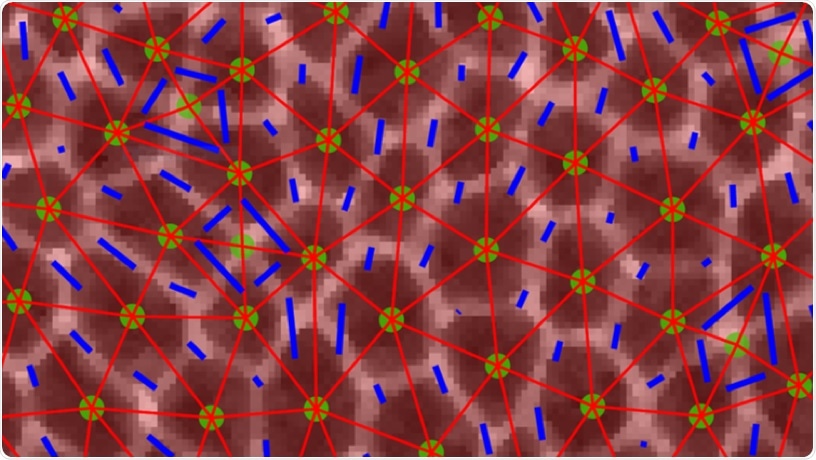
Confocal image of epithelial cells during dramatic tissue flows in the Drosophila embryo. Images of cells with overlaid triangles that were used to quantify anisotropy in the tissue. Image Credit: Columbia University School of Engineering and Applied Science.
When the embryo develops, these tissues usually narrow along one axis and extend along a perpendicular axis via cellular movement induced by internal or external forces that act differently along with numerous directions in the tissue (anisotropies).
For a long time, scientists have speculated how simple clusters of cells within the developing embryos change into organs and tissues—how do tissues physically alter the shape in the embryo?
And do they change from “solids” into “fluids” at certain times during the development to render it easier to quickly shape the functional organs and tissues?
Currently, it is not possible to watch and measure what occurs in tissues within the human embryo, and it is still very hard to perform this in mammalian models, such as mice.
Now researchers from Syracuse University and Columbia Engineering have decided to sort out this problem by targeting fruit flies because humans and the fruit fly Drosophila share several biological similarities.
In a study published online in the Proceedings of the National Academy of Sciences journal on May 29th, 2020, the researchers have reported that they can forecast when the tissue will start to quickly flow by simply watching the shapes of the cells in the tissue.
Thanks to earlier theoretical work from our colleagues at Syracuse, we thought we might be able to learn something about whether the embryonic tissues are solid or fluid by just looking at the shapes of cells in the tissue.”
Karen Kasza, Lead Principal Investigator and Clare Boothe Luce Assistant Professor of Mechanical Engineering, Columbia University
Kasza continued, “So we decided to try this in the fly. We’re really excited about our results, which could reveal fundamental mechanisms underlying human development and point to where things can go wrong, causing birth defects.”
The difficulty was how to apply standard engineering methods to quantify the mechanical properties of tissues and cells within the tiny embryos of the flies to observe the types of tissues that act like solids, retaining their shape and resisting flow, and also the types of tissues that behave like fluids, flowing effortlessly and altering their shapes.
The scientists employed high-resolution confocal fluorescence imaging to record films of embryonic development where they could observe in excellent detail the packings and shape of tissues and cells inside the fly embryo.
The scientists focused on an extremely rapid developmental event where the embryonic tissue quickly alters shape to extend the fly’s head-to-tail body axis (something that also occurs in a majority of the animal embryos).
The scientists at Columbia University combined experimental studies in the fruit fly embryo with the theoretical modeling approaches at Syracuse University and showed that the alignment and shapes of cells inside the tissues can help to both explain and predict the way tissues change their shapes during the development and how defects in such processes can lead to abnormalities in the shape of the embryo.
Kasza observed that “It was a fantastic collaboration between experiment and theory.”
From the theory side, it was really unclear what collective mechanisms allow the cells to easily rearrange during tissue elongation. With Professor Kasza’s group, who has some of the best tools in the world to study mechanical properties of fruit fly tissue, we were really able to nail down precisely how changes to cell shapes drive changes to tissue mechanics.”
Lisa Manning, Study Co-Author and William R. Kenan, Junior Professor of Physics, Syracuse University
Manning continued, “It is amazing that we can now just look at a snapshot of cell shapes in the fruit fly and predict how cells will move, with no fit parameters.”
To their amazement, the researchers could predict when the tissue would start to flow by simply watching the cell shapes in the tissue without making any modifiable parameters to the theoretical model.
However, unlike earlier studies and predictions, the researchers have to include a unique parameter, anisotropy, that explained the alignment of cells inside the tissue because the forces acting on and in the tissue were extremely anisotropic, or differed along with different directions in the tissue.
For the researchers, what was particularly fascinating was that their discoveries imply that embryonic tissue appears to become more fluid-like just before the rapid tissue flows take place during body axis elongation.
This is really exciting, because it suggests that the mechanical properties of the cells might be regulated biologically during embryonic development, i.e. in the genetic instructions encoded in DNA, to make it easier for tissues to change shape dramatically during brief time windows during development. This adds to a growing body of research revealing that mechanics is really crucial to understanding life.”
Karen Kasza, Lead Principal Investigator and Clare Boothe Luce Assistant Professor of Mechanical Engineering, Columbia University
Currently, the researchers are looking at how the instructions for variations in tissue fluidity are genetically encoded. The team is also investigating the mechanical properties of tissues to develop quantitative models of tissue morphogenesis that will allow them to design, build, predict, and manage the shape and movements of tissues in developing embryos as well as in engineered tissues in the laboratory.
Source:
Journal reference:
Wang, X., et al. (2020) Anisotropy links cell shapes to tissue flow during convergent extension. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.1916418117.