A clinical case of Japanese encephalitis is diagnosed in over 68,000 individuals every year, leading to the death of one in four of these affected patients.
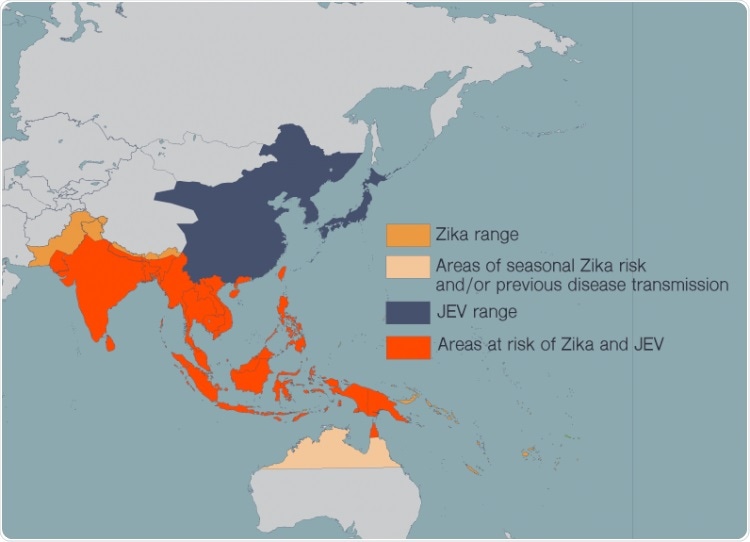
Zika, JEV, dengue, West Nile virus, and yellow fever have spread in recent years as more people around the world have moved to cities and climate change has allowed the mosquitoes that carry these diseases to expand their habitat. People in many countries now live at risk of encountering multiple harmful flaviviruses in their lives. Image Credit: Madeline McCurry-Schmidt, La Jolla Institute for Immunology.
Most common in Southeast Asia, the mosquito-borne virus also causes severe psychiatric disorders and neurological damage.
While there is no cure for Japanese encephalitis at present, effective vaccines are available against the Japanese encephalitis virus (JEV). The issue is that the range of JEV is spreading, and an increasing number of individuals at risk of JEV also reside in regions where viruses, such as Zika, are widespread.
In the latest study published in the Journal of Experimental Medicine on June 5th, 2020, researchers at La Jolla Institute for Immunology (LJI) have demonstrated that antibodies developed against JEV are not only “cross-reactive” but can also detect the Zika virus.
Regrettably, such antibodies can, in fact, make Zika cases more serious. The study, which was performed in mice, is the first in the world to demonstrate that this fatal phenomenon can be counteracted by T cells.
This means we probably need to be developing a vaccine against both viruses that can elicit a good balance of antibodies and T cells.”
Sujan Shresta, PhD, Study Co-Lead and Associate Professor, Center for Infectious Disease, La Jolla Institute for Immunology
Shresta performed the study in association with Jinsheng Wen, PhD, of Ningbo University and Wenzhou Medical University, and Yanjun Zhang, PhD, of Zhejiang Provincial Center for Disease Control and Prevention.
Shresta has devoted most of her career towards analyzing flaviruses—a group of viruses that includes dengue, JEV, Zika, yellow fever, and the West Nile virus.
In recent years, such diseases have spread extensively because more people across the world have migrated to cities, and climate change has also enabled the mosquitoes carrying these diseases to widen their habitat. In many nations, people currently live at risk of facing many dangerous flaviviruses in their lives.
The immune responses to these viruses are very cross-reactive. The problem is that the immune response can be both good and bad.”
Sujan Shresta, PhD, Study Co-Lead and Associate Professor, Center for Infectious Disease, La Jolla Institute for Immunology
In certain cases, antibodies against a single flavivirus can render an upcoming flavivirus infection more lethal by enabling the virus to penetrate the host cells.
Shresta and other researchers across the world have demonstrated this process, known as antibody-dependent enhancement (ADE), during dengue and Zika infections in animal models that summarize the severe cases of Zika or dengue disease in persons who were earlier exposed to either Zika or dengue virus.
But the ADE of Zika disease in cases of earlier exposure to JEV, and the interaction between antibodies and CD8+ T cells—infection-fighting immune cells—had not been analyzed in the past.
For the latest study, Shresta and her collaborators extracted antibodies from mice infected with JEV or people vaccinated with JEV and then administered them into healthy mice. They later exposed the healthy mice to the Zika virus. When compared to mice that lack antibodies against JEV, the mice injected with antibodies from JEV-infected animals experienced ADE and had much more severe cases of Zika fever.
Along with her collaborators, Shresta next targeted the CD8+ T cells extracted from JEV-infected mice. The researchers observed that CD8+ T cells primed to combat JEV could offset the dangerous impacts of cross-reactive antibodies.
“These JEV-elicited T cells were indeed able to recognize and get rid of the Zika virus infection,” added Shresta.
To sum it up, the survival rate of the mice increased and their viral load decreased because of the CD8+ T cells. A potential JEV vaccine would need to trigger an analogous reaction from CD8+ T cells to help an individual avoid the ADE of Zika infection.
According to Shresta, this study can provide a deeper understanding of how to combat the entire group of flaviviruses, which comprises more than 70 different species, and a majority of nations are increasingly tackling the co-circulation of numerous flaviviruses.
Any of these viruses could cause a major, major outbreak. We need to look at deploying a combination Zika/JEV vaccine, and we may need to tailor vaccines to particular locations where we know both JEV and Zika pose a threat.”
Sujan Shresta, PhD, Study Co-Lead and Associate Professor, Center for Infectious Disease, La Jolla Institute for Immunology
Shresta further added that studies into T cell responses and cross-reactive antibodies are particularly significant today, as researchers explore whether exposure to common cold coronaviruses can impart some kind of immunity to a person against the novel coronavirus—SARS-CoV-2.
“This provides us with a really good model to learn about immune response,” concluded Shresta.
Source:
Journal reference:
Chen, D., et al. (2020) Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis. Journal of Experimental Medicine. doi.org/10.1084/jem.20192152.