Metabolic processes are particularly complex in plants because of their obligate sessile lifestyle. This is the reason why researchers are discovering increasingly unique and unexpected connections that take place inside their cells.
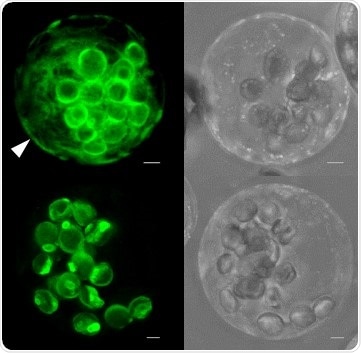
In transfected Arabidopsis protoplasts (plant cells without cell walls), green-fluorescent fusion proteins of GPT1 (GPT1-GFP, top) label both plastids and the endoplasmic reticulum (white arrow). This is not the case for GPT2 (GPT2-GFP, bottom). Image Credit: © M.-C. Baune et al./The Plant Cell/ASBP.
A significant metabolic pathway that has occupied botanists for many years is the supposed oxidative pentose-phosphate route through which carbohydrates are changed to reduction power. Two membrane proteins—GPT1 and GPT2—play a significant role in this metabolic pathway.
These proteins import activated glucose in the form of glucose-6-phosphate into plastids, which are unique cell organelles of plants, to “feed” the oxidative pentose-phosphate route. This is also a crucial process for the reproduction of plants, specifically during the development of ovules, pollens, and seeds.
Since indications were there that the three oxidative steps of the route may also take place in other cell organelles—that is, the peroxisomes—scientists from the University of Münster speculated whether membrane proteins are also directed concurrently to two different locations inside the same cell. Along with collaborators from the University of Düsseldorf, the researchers have now found an answer to this question.
The outcomes demonstrated that only the GPT1 protein is distributed to two sites—and the alternative pathway of the membrane protein leads through the endoplasmic reticulum, which is another net-like organelle of the cell. Therefore, the GPT1 protein simultaneously enables the formation of reduction power at two locations, that is, the capability to convey electrons.
Our study shows that besides in plastids and the cytoplasm, the oxidative pentose-phosphate pathway is also a main source of the energy-rich coenzyme NADPH in peroxisomes.”
Antje von Schaewen, Study Lead and Professor, University of Münster
The scientists believe that plants in which this route is blocked in peroxisomes are less likely to be resistant to stress. The study was published in The Plant Cell journal.
Background and method
The researchers studied the processes in Arabidopsis thaliana—a genetic model plant. They have already demonstrated in earlier studies how other enzymes of the route in this species are re-directed from the cytoplasm or plastids to peroxisomes under specific conditions.
To accomplish this, scientists used fluorescent reporter fusion proteins. This technique was also employed in their present research to observe the GPT1 and GPT2 proteins through contemporary light microscopic and life-cell imaging techniques.
The researchers observed that only the GPT1 protein targets both the endoplasmic reticulum and plastids, from which new peroxisomes are developed in a specific region. The researchers noticed that a still-unidentified factor initially inhibits the transport of the GPT1 protein to peroxisomes—until the membrane protein is required there.
By experimentally enforcing interaction with peroxisomal import proteins at the endoplasmic reticulum we could show that GPT1 can be ‘dragged’ to its alternative location.”
Dr Marie-Christin Baune, Study First Author, University of Münster
When the scientists found that the GPT1 protein occurs at peroxisomal membranes, they analyzed an artificially reconstituted system in yeast, in which transport processes may take place. The scientists found that the GPT1 protein prefers to trade off glucose-6-phosphate for ribulose-5-phosphate—the product which again exits the organelle. Ribulose-5-phosphate is known to be a significant precursor of nucleotides—the building blocks of nucleic acids.
The supposed immunoblot analyses through which proteins are observed further implied that the GPT1 protein is crucial at plastids in fertilization and also at peroxisomes. Therefore, the GPT1 protein varies significantly from its “brother” GPT2—an outcome that the researchers had not anticipated and which also became obvious in genetically modified plants.
Our results suggest that GPT1 and GPT2, despite their high similarity, fulfill only minimally overlapping tasks in plants. The loss of GPT2 is tolerated, at least under laboratory conditions. GPT1 however is indispensable, both at plastids and peroxisomes.”
Dr Hannes Lansing, Study Co-Author, University of Münster
“Since we now know that all reactions of the oxidative pentose-phosphate pathway that produce NADPH and ribulose-5-phosphate may occur in peroxisomes, we want to find out in future studies which other processes depend on them,” concluded Antje von Schaewen.
Source:
Journal reference:
Baune, M.-C., et al. (2020) The Arabidopsis Plastidial Glucose-6-Phosphate Transporter GPT1 is Dually Targeted to Peroxisomes via the Endoplasmic Reticulum. The Plant Cell. doi.org/10.1105/tpc.19.00959.