A recent study that closely analyzed the cardiac health of flies offers new evidence that dysfunction in the liver may result in the deterioration of the heart.
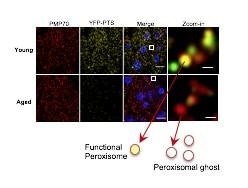
Peroxisomal protein import is significantly impaired in aged fly hepatocytes. The number of peroxisomes (Red fluorescence) does not change, whereas peroxisomal protein import (Yellow fluorescence) decreases with age. The relationship among peroxisomes, liver function, and heart aging might become a promising target for new therapies. Image Credit: Hua Bai.
The study fills the existing gaps in how researchers interpret the connections between heart health and other types of tissues and may potentially lead to the development of novel therapies in human medicine, stated Hua Bai, an Assistant Professor of Genetics, Development and Cell Biology at Iowa State University.
Bai’s laboratory had earlier carried out studies on how cardiac health in flies alters with age. Published in the academic journal Nature Communications, the latest study also spans new ground in the function of a poorly understood organelle known as the peroxisome, which is likely to play a significant role in the aging of organisms.
We were thinking outside the heart for this paper, we wanted to find out if other tissues affect cardiac function during aging. There is significant data suggesting that liver function actually is a risk factor for cardiac disease. A patient with a lot of liver dysfunction often develops cardiac disease. This is a concern because you may have two diseases that you have to deal with for these patients.”
Hua Bai, Assistant Professor, Department of Genetics, Development, and Cell Biology, Iowa State University
But according to Bai, experiments have not revealed any direct association between heart disease and liver dysfunction, leaving medical professionals uncertain of whether the two factors share an informal association or whether there is merely a correlation.
Bai’s laboratory tried to fill that gap by analyzing the communication between liver disease and the cardiac muscle function in flies.
Protecting the liver maintains heart health
Earlier studies conducted by Bai’s laboratory revealed that genes in the flies’ cardiac muscles could be manipulated to restore the heart function of older flies to a state analogous to younger flies, fundamentally turning back the clock on cardiac tissues.
In recent experiments, the scientists exploited numerous genes that control liver function in the flies to observe how that would impact the heart health of these insects as they aged.
Our findings demonstrate we can protect the liver of old animals and maintain the health of the heart without doing any direct intervention on the heart tissue.”
Kerui Huang, Study Lead Author, Iowa State University
Huang is a graduate student in Bai’s laboratory.
Most of the genetic work performed by the scientists targeted the peroxisomes—understudied organelles found within cells that control major lipid metabolic processes and detoxification crucial for liver and brain function.
Looking at all the biology literature, we don’t know much about how peroxisome function changes in aged animals, we show that peroxisomal protein import function is significantly impaired in aged flies. Research like ours could open up another new field to study how peroxisomes regulate tissue aging.”
Hua Bai, Assistant Professor, Department of Genetics, Development, and Cell Biology, Iowa State University
Although flies seem to be highly dissimilar to humans, human medicine can still gain much by studying fly biology, added Huang. For example, the functions of a fly’s heart and liver share several analogous functions with the human heart and liver.
Pharmaceutical firms have shown a keen interest in exploring new opportunities to treat age-related diseases. The link between heart aging, liver function, and peroxisomes explained in the latest study might become a potential target for new drugs and therapies, Huang concluded.
Source:
Journal reference:
Huang, K., et al. (2020) Impaired peroxisomal import in Drosophila oenocytes causes cardiac dysfunction by inducing upd3 as a peroxikine. Nature Communications. doi.org/10.1038/s41467-020-16781-w.