The brain is known to contain two types of cells—the glial cells and the neurons. The glial cells receive relatively less attention for their significance in the function and disease of the brain than the more popular neurons.
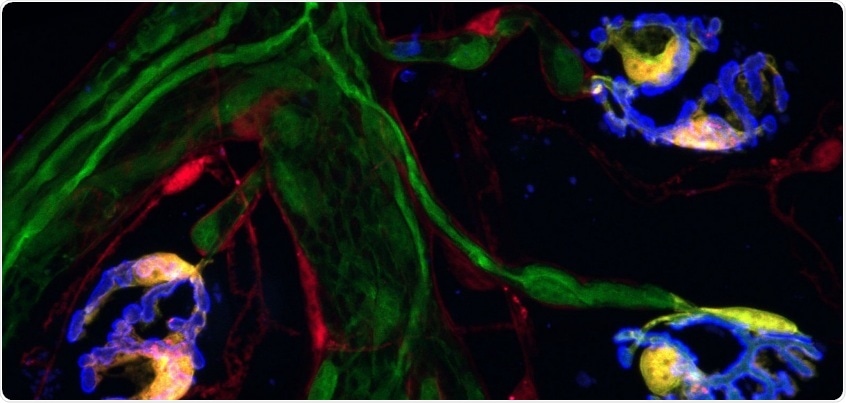
Two molecular markers, indicated by the red and green fluorescence, are found together only in synaptic Schwann cells. Together they offer a “bar code” that identifies Schwann cells, an important subtype of glia. Image Credit: Valdez Lab/Center for Translational Neuroscience/Brown University.
However, for over 100 years, researchers have known that unique types of glial cells are crucial components of synapses or neuromuscular junctions—points of contact between muscles and neurons that allow the brain to regulate movements.
Regardless of the importance of glial cells for the precise formation, repair, and maintenance of synapses all through the nervous system, the failure to differentiate particular glial cells at synapses from the varied and overall population of glial cells poses a significant challenge in supporting and restoring the normal activity of the nervous system in old age, and following diseases and injuries.
But that might be changed by a recent discovery described in the eLife journal on June 25th, 2020.
A research team, headed by Gregorio Valdez, Associate Professor of Molecular Biology, Cell Biology, and Biochemistry from Brown University, has detected crucial molecules to analyze and exploit the particular glial cells that are essential to synapses.
This discovery will serve as a springboard to addressing fundamental questions and developing assays to speed the discovery of therapeutics intended to preserve and restore the normal function of neuronal circuits.”
Gregorio Valdez, Associate Professor, Department of Molecular Biology, Cell Biology and Biochemistry, Brown University
Valdez is also affiliated with the new Center for Translational Neuroscience, founded by the Carney Institute for Brain Science at Brown University and the Brown Institute for Translational Science.
The study shows that a significant subtype of glia, called Schwann cells and situated at neuromuscular synapses, are the only cells in muscles that express a pair of specific molecules. Such molecular markers offer an extremely specific glial “bar code,” which according to Valdez detects the crucial cell subtype.
What this means is that we can finally figure out how all three cellular constituents of the synapse—neurons, muscle and glia—talk to each other. We now have a unique and important tool for identifying this critical component of the synapse. This is essential for knowing when and where to target to ensure synapses function appropriately.”
Gregorio Valdez, Associate Professor, Department of Molecular Biology, Cell Biology and Biochemistry, Brown University
According to Valdez, the innovative bar code tool will present new opportunities for upcoming studies, including neuromuscular diseases, like spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).
Researchers can employ the molecular markers to investigate the function of synaptic glia in neuromuscular synapse repair after injury, the progression of neuromuscular diseases, and the degeneration that occurs during normal aging.
Valdez also believes that an analogous method will expose the synaptic glial cells situated at synapses between pairs of neurons found in the brain.
While our primary focus was the neuromuscular synapse, we also gathered initial evidence indicating that synaptic glia cells in the brain can be labeled and targeted using the same approach. If true, this discovery could be of immense consequence for treating a myriad of brain conditions, including those involving cognitive decline due to normal aging and Alzheimer’s disease.”
Gregorio Valdez, Associate Professor, Department of Molecular Biology, Cell Biology and Biochemistry, Brown University
Source:
Journal reference:
Castro, R., et al. (2020) Specific labeling of synaptic Schwann cells reveals unique cellular and molecular features. eLife. doi.org/10.7554/eLife.56935.