Millions of individuals suffer from Alzheimer’s disease (AD). Neurons in the brains of such people are being depleted gradually and inevitably, resulting in the characteristic loss of cognitive function and memory related to this medical condition.
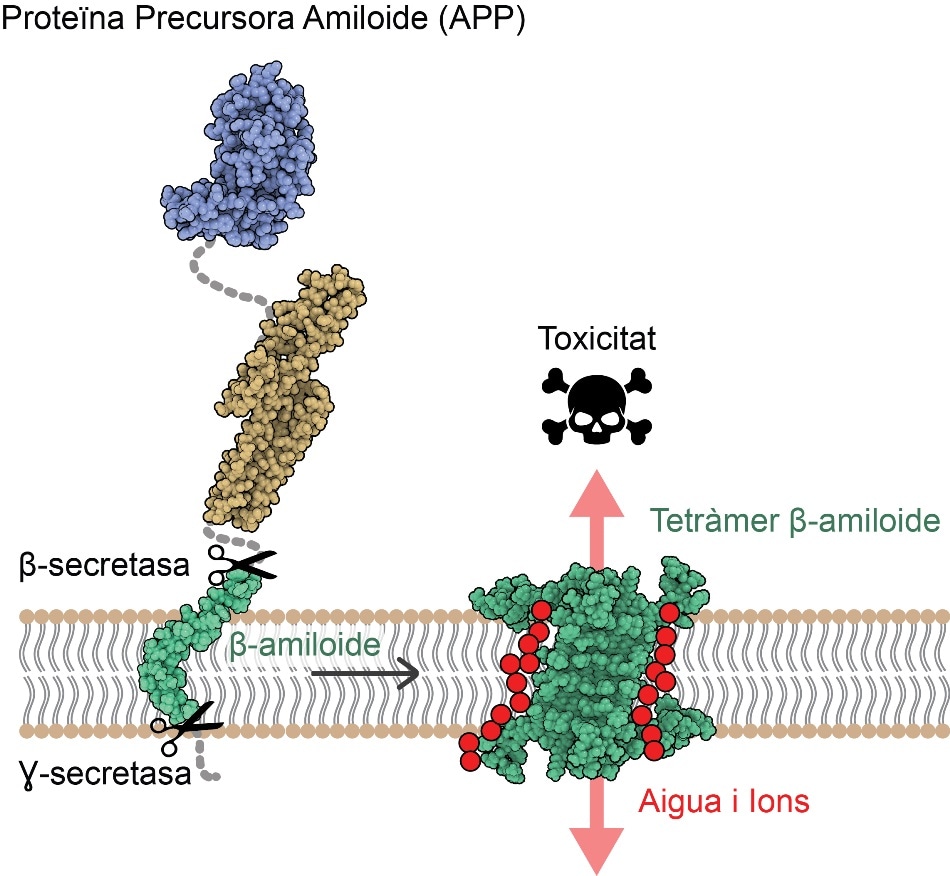
After APP cleavage at the neuronal membrane, Aβ protein self-assembles into 4 or 8 copies adopting an arrangement allowing passage of water and ions (in red) and thus disrupting ion cell homeostasis. Image Credit: © Benjamin Bardiaux.
But the cause of neuronal death still remains elusive. The aim of the existing treatments is to slow down the progression of dementia and help enhance the quality of life for brief periods. Hence, treatments to essentially cure AD continue to be an unmet medical requirement.
For the first time, scientists have exposed the atomic structure of the assemblies of amyloid-beta (Aβ) protein. The team was headed by Natalia Carulla, an Alumni of Institute for Research in Biomedicine (IRB Barcelona) and a former group leader at the Institut Européen de Chimie et Biologie (IECB) based in Bordeaux. Carulla is presently a project manager at Grup CIEF.
The understanding of this atomic structure exposes a unique mechanism of toxicity for such assemblies, with the potential to disturb the neuronal membrane, enabling ions and water to permeate it and leading to cell death.
Many studies have suggested that the communication between the neuronal membrane and the Aβ protein accounts for the neuronal death noticed in AD. But the Aβ protein is a hard therapeutic target because it is not only “sticky” but also assembles on its own, adopting clear sizes and shapes.
Knowing the features that characterize these protein ensembles, such as the number of molecules that make them and the shape they adopt, is crucial to design effective therapeutic strategies that target the forms of Aβ ensembles responsible for the neurotoxicity in AD.”
Natalia Carulla, Project Manager, Grup CIEF
An in vitro approach to ensure stable Aβ forms
To overcome the instability of the various conformations, the researchers initially analyzed the Aβ protein in vitro—in streamlined model systems that imitate the membrane of the neuron—to create conditions to prepare stable forms of Aβ that have uniform shape and composition.
Upon identifying the different compositions, the researchers analyzed their mode of neurotoxicity and structure and established a three-dimensional (3D) arrangement of all the atoms that constitute the Aβ ensemble.
According to Sonia Ciudad and Eduard Puig, the study’s first authors, “Our study suggests that some Aβ associations can perforate the membrane of neurons, alter their osmotic equilibrium, and consequently trigger their death.”
Ciudad is also an Alumni of IRB Barcelona and presently an R&D Scientist at Biokit—a Werfen Company—while Puig is currently a postdoctoral fellow in Research Unit on Asymmetric Synthesis at IRB Barcelona.
Targeting membrane pores to avoid neurotoxicity
The new study has emphasized a pair of Aβ protein ensembles, one created by four Aβ proteins and the other formed by eight Aβ proteins. The protein ensembles are arranged such that they can disrupt the cell membrane, indicating them as candidates for promoting neurodegeneration in AD.
Additional work should target methods to avoid the formation of this ensemble, thereby inhibiting the disruption of membranes. At present, the drug discovery pipeline in this area lacks any membrane-associated, drug-targeting Aβ assemblies, so this discovery could make a major advancement in AD treatment.
Source:
Journal reference:
Ciudad, S., et al. (2020) Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage. Nature Communications. doi.org/10.1038/s41467-020-16566-1.