In people affected by Parkinson’s disease, clusters of alpha-synuclein or α-synuclein, also referred to as the “Parkinson’s protein,” are detected in the brain. These clusters of proteins kill cell membranes, which may ultimately lead to cell death.
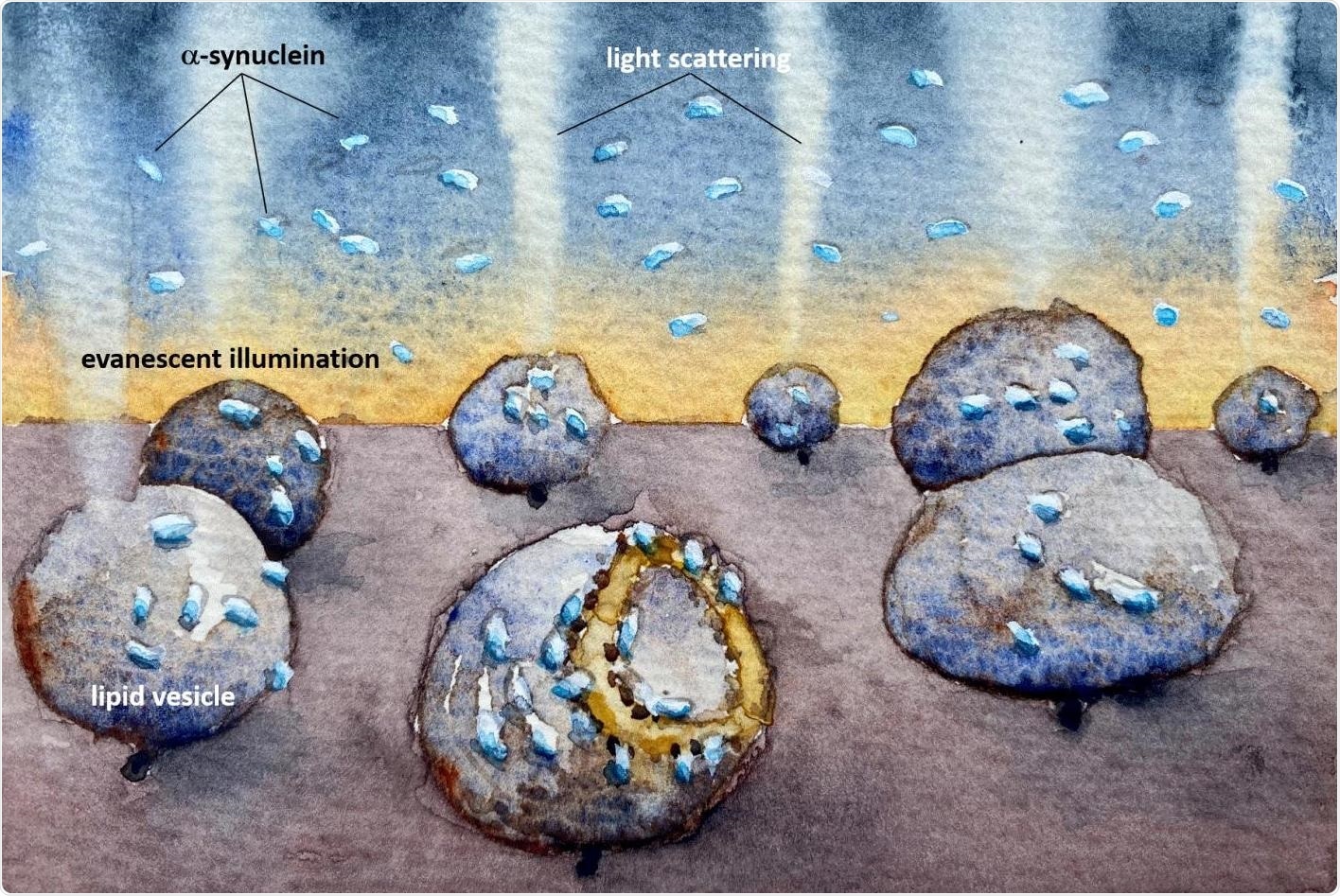
An illustration showing how the mitochondria-imitating lipid vesicles are damaged by the Parkinson’s protein alpha synuclein. The light scattering reveals how the membrane is destroyed even at very low, nanomolar, concentrations, where proteins do not aggregate to clumps before they bind. The watercolor illustration was created by Fredrik Höök. Image Credit: Fredrik Höök/Chalmers University of Technology.
Recently, researchers from the Chalmers University of Technology based in Sweden have devised a novel approach that shows how the cell membrane’s composition appears to be a crucial factor in the extent of damage caused by small amounts of α-synuclein.
Parkinson’s disease is regarded as an incurable medical condition, where the nerve cells or neurons in the brain slowly disintegrate, affecting the functions of the brain.
This medical condition includes symptoms like involuntary shaking of the body and can cause great distress to the affected patients. To design drugs that stop or slow down the disease, scientists try to interpret the molecular mechanisms behind the role played by α-synuclein in neuronal degeneration.
Researchers know that mitochondria—the energy-producing compartments found in cells—are damaged in Parkinson’s disease, perhaps due to “amyloids” of α-synuclein.
Amyloids are essentially a cluster of proteins organized into extended fibers with a well-ordered core structure. The formation of these amyloids underlies a number of neurodegenerative diseases.
While amyloids or even smaller clusters of α-synuclein can probably adhere to and destroy the membranes of mitochondria, the precise mechanisms are yet to be fully understood.
The latest study, which was recently published in the Proceedings of the National Academy of Sciences, targets two different kinds of membrane-like vesicles. Vesicles are “capsules” of lipids that can be utilized as mimics of the membranes within the cells.
While one vesicle is composed of lipids that are usually located in the synaptic vesicles, the other is made up of lipids associated with mitochondrial membranes.
The scientists discovered that the Parkinson’s protein would adhere to both types of vesicles, but it would only make structural modifications to the mitochondrial-like vesicles, which leak their contents by deforming asymmetrically.
Now we have developed a method which is sensitive enough to observe how α-synuclein interacts with individual model vesicles. In our study, we observed that α-synuclein binds to—and destroys—mitochondrial-like membranes, but there was no destruction of the membranes of synaptic-like vesicles.”
Pernilla Wittung-Stafshede, Professor, Department of Biology and Biological Engineering, Chalmers University of Technology
Wittung-Stafshede continued, “The damage occurs at very low, nanomolar concentration, where the protein is only present as monomers—non-aggregated proteins. Such low protein concentration has been hard to study before but the reactions we have detected now could be a crucial step in the course of the disease.”
The novel approach developed by the research team from the Chalmers University of Technology makes it viable to analyze small quantities of biological molecules without the use of fluorescent markers. This offers an excellent benefit when monitoring natural reactions since the markers usually impact the reactions one wants to visualize, particularly when working with small proteins like α-synuclein.
The chemical differences between the two lipids used are very small, but still we observed dramatic differences in how α-synuclein affected the different vesicle. We believe that lipid chemistry is not the only determining factor, but also that there are macroscopic differences between the two membranes—such as the dynamics and interactions between the lipids. No one has really looked closely at what happens to the membrane itself when α-synuclein binds to it, and never at these low concentrations.”
Pernilla Wittung-Stafshede, Professor, Department of Biology and Biological Engineering, Chalmers University of Technology
The subsequent step for the scientists is to analyze the variants of the α-synuclein protein with mutations linked with Parkinson’s disease, and also to study lipid vesicles that are more analogous to cell membranes.
We also want to perform quantitative analyses to understand, at a mechanistic level, how individual proteins gathering on the surface of the membrane can cause damage.”
Fredrik Hook, Researcher and Professor, Department of Physics, Chalmers University of Technology
“Our vision is to further refine the method so that we can study not only individual, small—100 nanometres—lipid vesicles, but also track each protein one by one, even though they are only 1-2 nanometres in size. That would help us reveal how small variations in properties of lipid membranes contribute to such a different response to protein binding as we now observed,” Hook concluded.
Source:
Journal reference:
Hannestad, J. K., et al. (2020) Single-vesicle imaging reveals lipid-selective and stepwise membrane disruption by monomeric α-synuclein. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.1914670117.