Researchers have unraveled a crucial factor that governs whether malignant cells will develop into a tumor. The study was recently published in the eLife journal.
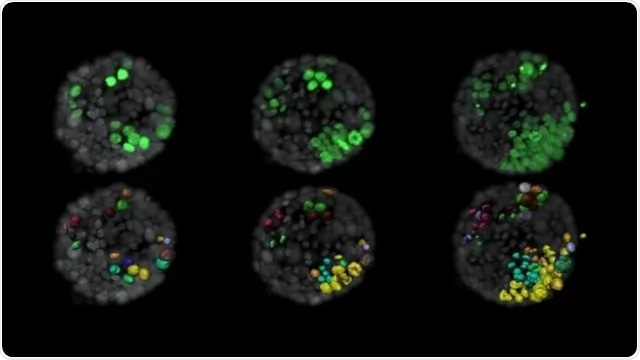
Evolution of tumor cells (green) within a normal organoid (grey). The lower panel shows the surface rendition of tumor cells and labels new cells that arise from a single cell in the same color. Image Credit: Ashna Alladin.
In this study, the researchers used a combination of mouse breast mini-organs along with continuous imaging at single-cell resolution to expose the way a tumor becomes established. The latest findings shed light on the behavior of individual breast cancer cells that may result in new methods for controlling the disease.
A majority of the research works focused on cancer biology make use of cells or tissue samples in which each cell has defective mutations. Such mutations could develop into a tumor. In other words, it is not possible to reproduce the earliest stages of the disorder when a single mutated cell overcomes its usual environments and turns into cancer.
To study tumor initiation, models need to be established where only a few malignant cells expand in the context of their immediate non-tumor environment. We created a model of breast cancer development where only single cells activate cancer-promoting oncogenes within otherwise healthy breast tissue, and then followed the fate of these cancerous cells.”
Ashna Alladin, Study Lead Author, European Molecular Biology Laboratory
Alladin is also a postdoctoral researcher in senior author Martin Jechlinger’s laboratory at the European Molecular Biology Laboratory.
The researchers developed their model by growing mini-organs or organoids from the mouse breast tissue, which replicate the structure of the breast.
The researchers targeted the small glands, known as “acini,” inside the breast and subsequently introduced a cancer-promoting gene into certain individual cells inside these acini. A second gene was then added to some of the cells, and this enabled the cancer-promoting gene to be turned on in the presence of a drug known as doxorubicin.
This means the researchers could regulate the types of cells in which the cancer-promoting gene is turned on and “transformed” into malignant cells, allowing them to record when the cells are actually converted.
The team also labeled the cells, so that they could be monitored separately every 10 minutes for up to four days through a technique known as light-sheet microscopy. In this manner, the researchers could precisely observe which kinds of cells continued to stimulate a tumor, and which ones did not.
To find out the factors that make the individual malignant cells to form into a tumor, the scientists developed a computer algorithm that took the entire data from the cell images and searched for general features related to cells that continued to form tumors.
Of all the traits examined, only one was considerably associated with the development of a tumor—the number of cells comprising the cancer-promoting gene inside a tumor. For each extra cell in a cluster containing a cancer-promoting gene, the chances of this cluster developing into a tumor increased by as much as nine times.
Based on the modeling method, the researchers examined the acini images and observed that cells that continued to establish tumors toward the end of the four-day imaging period appear to cluster together at the beginning of the imaging period. On the other hand, cells that did not form tumors were located more sparsely.
Our results suggest that there is some interaction or signaling that occurs between malignant cells in close proximity that guides the initiation of a tumor within normal tissues. Our ability to image the fate of these malignant single cells among healthy tissue means that it is now possible to follow tumor initiation, growth and treatment via inhibition by drugs in a realistic model, thereby helping to bridge the gap between model systems and the clinic.”
Martin Jechlinger, Study Senior Author and Group Leader, European Molecular Biology Laboratory
Source:
Journal reference:
Alladin, A., et al. (2020) Tracking cells in epithelial acini by light sheet microscopy reveals proximity effects in breast cancer initiation. eLife. doi.org/10.7554/eLife.54066.