A team of researchers from the Tokyo Institute of Technology and the Institute of Microbial Chemistry has analyzed the degradation mechanism of the endoplasmic reticulum (ER) called ER-phagy.
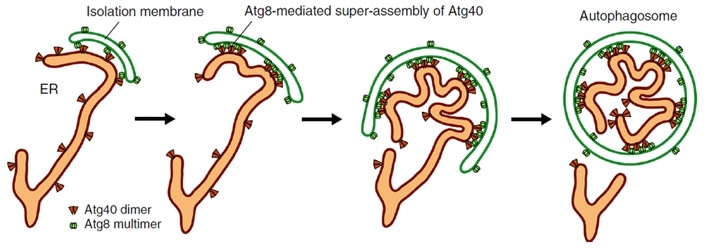
Atg40 binds to Atg8 on the isolation membrane. This process changes ER membrane shape so that it can be packed into the isolation membrane, forming an autophagosome that then degrades the ER parts. Image Credit: Nature Communications, Tokyo Institute of Technology.
Endoplasmic reticulum is a crucial organelle that has numerous biologically required functions, such as the production of lipids and proteins.
Degradation is essential for preserving the functions of the ER. Researchers have discovered that the “Atg40” protein marks the parts of the ER to be degraded by autophagy and also folds them to facilitate efficient degradation. This provides a deeper insight into a crucial process involved in the maintenance of cells.
The ER is a significant part of eukaryotic cells (cell types that constitute every living thing other than viruses or bacteria, including humans). These cells are a cluster of tubes linked to the cell nucleus; both lipids and proteins are synthesized in the ER networks.
For this ER to function efficiently, cells often degrade parts of the ER to promote its renewal. Such a process is known as ER-phagy or ER autophagy, in which a structure known as “isolation membrane” expands and then closes up to create an “autophagosome.” The closure mechanism separates numerous cellular materials including ER inside the autophagosome, which subsequently carries the waste away for degradation.
While this can be a random process, researchers have unraveled “autophagy receptors” that attach particularly to specific targets and communicate with a class of proteins known as Atg8, situated on the isolation membrane.
Such an interaction enables cells to target particular parts for degradation. In the case of yeast, an organism that is often used for biological studies, researchers discovered that the Atg40 protein is an ER-phagy receptor, and they also discovered that parts of this protein structure share similarities with a class of proteins known as DP1/Yop1 (that is, reticulon-like proteins), which “curves” the ER membranes into shape and preserves their tubular structures.
Our previous work reveals that Atg40 is important for ER-phagy, but we actually know very little about how the process works. Because degradation of ER is so important for proper cellular function, gaining a better understanding of ER-phagy will improve basic biological knowledge.”
Dr Hitoshi Nakatogawa, Team Leader, Tokyo Institute of Technology
A research team, headed by Dr Nakatogawa, studied the mechanisms of Atg40 involvement in ER-phagy.
The yeast experiments, the results of which were reported in the Nature Communications journal, demonstrated that the Atg40 protein is crucial for “curving and folding” the ER membrane, and hence has a function analogous to DP1/Yop1, describing their structural similarities.
The Atg40 protein is also essential for ER-phagy, particularly contributing to the disintegration of the ER membrane so that it can be imbibed by autophagosomes. The scientists showed that when the ER folds and disintegrates, the Atg40 protein forms an assembly of proteins (protein cluster) by communicating with Atg8 located particularly at points of contact between the isolation membrane and the ER, as shown in the above image.
In a nutshell, the Atg40 protein does not arbitrarily or invariably remodel the structure of the ER, and it does so only for the parts of the ER that will be degraded.
With regard to the importance of these outcomes, Dr Nakatogawa added, “What I find particular exciting is the insight we gained on a crucial part of how cells work, how they deal with waste or get rid of abnormal cell parts.”
Our work doesn’t just have implications for ER-phagy though, it can also potentially tell us something about how other organelles, like the nucleus or mitochondria, are degraded.”
Dr Hitoshi Nakatogawa, Team Leader, Tokyo Institute of Technology
Apart from being valuable fundamental research, the study results also have important practical applications. Understanding the mechanisms of the degradation of the organelles may help develop drugs that focus on this process if it disintegrates. This presents promising attractive solutions for various diseases involving the malfunction of ER, like sensory neuropathy.
Source:
Journal reference:
Mochida, K., et al. (2020) Super-assembly of ER-phagy receptor Atg40 induces local ER remodeling at contacts with forming autophagosomal membranes. Nature Communications. doi.org/10.1038/s41467-020-17163-y.