Using a detailed genetic analysis, a research team from Japan-based Kumamoto University has discovered that the NSD2 enzyme, which controls the actions of several genes, also functions to block the aging of cells.
The human body and the cells that make it up have a “program” for aging. It is thought that there is an accumulation of senescent cells in tissues and organs as we get older. Image Credit: Professor Mitsuyoshi Nakao.
The researchers’ experiments demonstrated that the (1) suppression of the function of NSD2 enzyme in normal cells results in rapid senescence and (2) that there is a striking reduction in the level of the NSD2 enzyme found in senescent cells.
The team believes that these latest discoveries will help explain the aging mechanisms, age-related pathophysiology, and the advancement of control techniques for maintaining the functionality of the NSD2 enzyme.
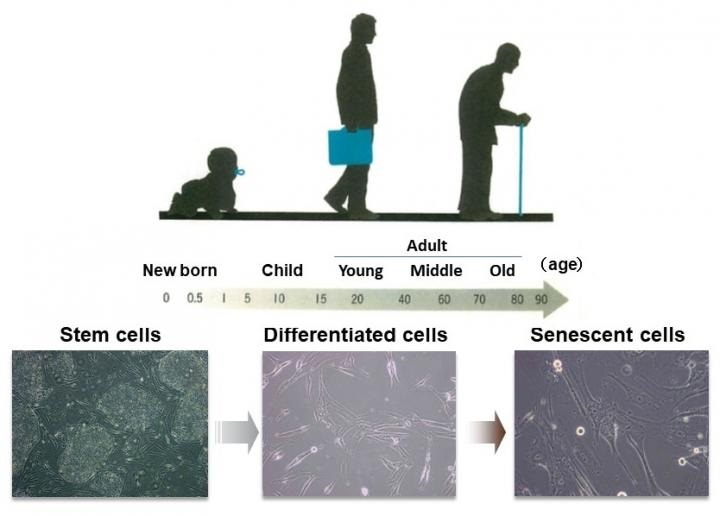
When the body’s cells continue to divide (reproduction of cells), their function ultimately decreases and they cease to grow. A cellular senescence like this is a significant factor in longevity and health.
Cell aging can also be activated when genomic DNA is impaired by physical stress, caused by ultraviolet rays or any other radiation, or by chemical stress due to specific kinds of drugs. But the in-depth aging mechanisms are still unknown.
Cell aging can be useful when a particular cell turns out to be cancerous; it inhibits malignant modifications by inducing cellular senescence. However, it may also lead to several diseases with age. Hence, it is crucial to precisely regulate cell aging.
Even though senescent cells tend to lose their proliferative potential, it has now become evident that senescent cells release numerous proteins that act on surrounding cells to support cancer development and chronic inflammation. As senescent cells are more active than anticipated, cellular aging is believed to be responsible for aging in the whole body.
This concept has been supported by reports of systemic aging suppression performed in aged mice following the elimination of accumulated senescent cells. To put this in simple terms, if individuals can regulate cell aging, they might be able to regulate the advancement of aging all through the body.
When an oncogene is excited and starts to become cancerous, cellular senescence takes place to prevent this process. Earlier, Kumamoto University researchers had reported that senescent cells considerably boosted mitochondrial metabolic functions, and that the SETD8 methyltransferase enzyme inhibits cellular aging. Here, the team found that NSD2 methyltransferase also has a role to play in inhibiting senescence.
NSD2 was previously demonstrated to control the function of genes. Moreover, it was believed that methylation by NSD2 of the histone proteins enclosed around genomic DNA improved the role of genes in the vicinity. But scientists were unaware of its association with cell aging.
Applying extensive genetic screening to inhibit the action of the NSD2 gene in fibroblasts while conducting knockdown (RNA interference technique), the researchers induced cell senescence which revealed the typical features of the senescent cells. This means the team had discovered that NSD2 plays a crucial role in inhibiting cell senescence.
Using mRNA sequencing, the researchers extensively examined all protein-coding gene expressions to study senescent cells that have reduced NSD2. The gene expression associated with cell aging increased and, specifically, the role of protein genes that support cell growth reduced.
Histones situated in such gene clusters are methylated by the NSD2 enzyme in proliferating cells; however, methylation appeared to reduce in senescent cells that have decreased NSD2. In other words, reduced NSD2 decreases the activities of genes that play a role in cell growth, thus stopping growth.
The team subsequently performed serum response experiments to check the regulation of the NSD2 enzyme. Cells usually grow by the actions of proteins that support growth (or growth factors) in serum. By contrast, senescent cells permanently cease to proliferate and do not usually increase again.
The experiment demonstrated that the inclusion of serum quickly raised the amount of the NSD2 enzyme, and that NSD2 is required for the expression of cell growth and growth-promoting genes. It was also observed that senescent cells with reduced NSD2 fully lack the potential to grow in serum. Therefore, the NSD2 enzyme is believed to inhibit cell senescence by sustaining serum response as well as cell growth
NSD2 is the fourth protective factor of cellular senescence that our team has identified. With the discovery that NSD2 protects against cellular senescence, this study clarifies a basic mechanism of aging. We expect this to be useful for elucidating aging mechanisms and developing control methods to regulate enzyme activity by chemicals or metabolites.”
Mitsuyoshi Nakao, Study Corresponding Author and Professor, Department of Medical Cell Biology, Institute of Molecular Embryology and Genetics, Kumamoto University
Source:
Journal reference:
Tanaka, H., et al. (2020) The NSD2/WHSC1/MMSET methyltransferase prevents cellular senescence‐associated epigenomic remodeling. Aging Cell. doi.org/10.1111/acel.13173.