The life of each human being starts from a single cell, which subsequently divides to ultimately form into an embryo. Based on the signals transmitted by their neighboring cells, such divided cells are subsequently differentiated or develop into particular organs or tissues.
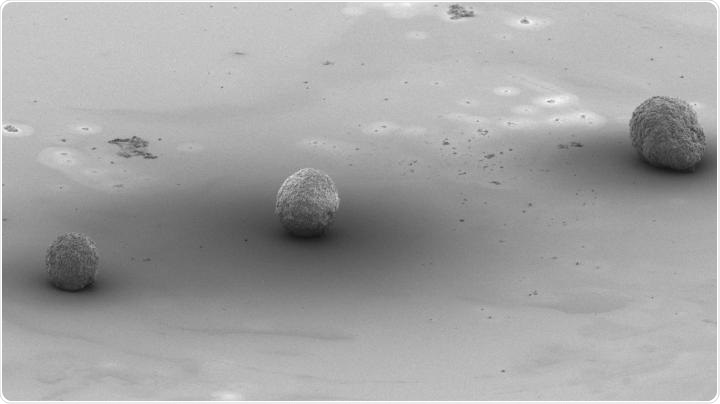
Artificial embryoid bodies with different characteristics which were made using 3D printing. Image Credit: Singapore University of Technology and Design.
In the field of regenerative medicine, regulating that differentiation in a laboratory setting is very important because stem cells could be differentiated to enable the growth of organs in vitro and substitute impaired adult cells, especially those that have very limited potential to replicate, for example, the heart or brain.
When it comes to differentiating stem cells, scientists often use chemical stimulators—a method that is quite common. Although this technique is highly efficient for producing a single type of cells, it does not have the potential to recreate the complexity of living organisms, in which many types of cells coexist and bind to develop into an organ.
There is also another method that was inspired by the natural process of the development of cells. In this method, the stem cells are packed into tiny cellular spheres, or aggregates, known as embryoid bodies.
Just like real embryos, the interaction between the cells in the embryoid bodies is the key driver of differentiation. Based on the synthesis of such embryoid bodies, it was observed that parameters like cell size, numbers, as well as sphericity of the embryoid body affected the types of cells that are created.
But since researchers were unable to regulate those parameters, they were forced to arduously create huge numbers of embryoid bodies and choose particular ones with appropriate traits to be analyzed.
To deal with this problem, a group of scientists from the Singapore University of Technology and Design (SUTD) looked at additive manufacturing to regulate the differentiation of stem cells in embryoid bodies. The study results were published in the Bioprinting journal.
PhD student Rupambika Das and Assistant Professor Javier G. Fernandez used a multidisciplinary method by integrating the research areas of life sciences and 3D manufacturing and successfully 3D printed a number of micro-scaled physical devices with finely adjusted geometries.
The team used the devices to show unparalleled accuracy in the directed stem cell differentiation via the formation of embryoid bodies, as depicted in the above image. In this research, the team was able to control the parameters required to improve the production of cardiomyocytes—that is, cells in the heart.
The field of additive manufacturing is evolving at an unrivaled pace. We are seeing levels of precision, speed and cost that were inconceivable just a few years ago. What we have demonstrated is that 3D printing has now reached the point of geometrical accuracy where it is able to control the outcome of stem cell differentiation.”
Javier G. Fernandez, Principal Investigator and Assistant Professor, Singapore University of Technology and Design
Fernandez continued, “And in doing so, we are propelling regenerative medicine to further advance alongside the accelerated rate of the additive manufacturing industry.”
The use of 3D printing in biology has been strongly focused on the printing of artificial tissues using cell laden cells, to build artificial organs ‘piece by piece’. Now, we have demonstrated that 3D printing has the potential for it to be used in a bio-inspired approach in which we can control cells to grow in a lab just as they grow in vivo.”
Rupambika Das, Study First Author and PhD Student, Singapore University of Technology and Design
Source:
Journal reference:
Das, R & Fernandez, J G (2020) Additive manufacturing enables production of de novo cardiomyocytes by controlling embryoid body aggregation. Bioprinting. doi.org/10.1016/j.bprint.2020.e00091.