The tiny organelles mitochondria supply the energy crucial for all cells in the human body, specifically in the brain that consumes an excessive amount of fuel.
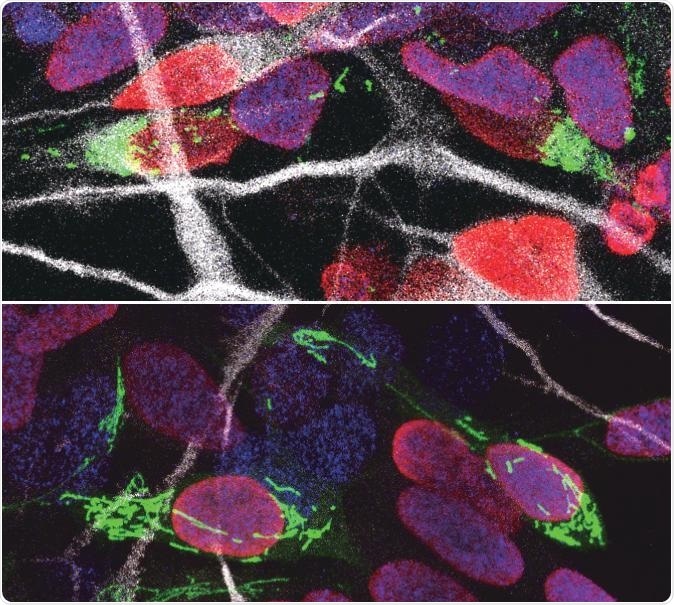
Human progenitor cells with their DNA-containing nucleus color red after division and their mitochondria labeled in green. Human cells with fragmented mitochondria (top) became neurons (top), whereas those with tubular mitochondria (bottom) remained progenitors. Progenitor cells have their DNA-containing nucleus marked with blue while new born neurons are marked in white. Image Credit: VIB (The Flanders Institute for Biotechnology)—Ryohei Iwata.
In the latest edition of the Science journal, a Belgian research team headed by Pierre Vanderhaeghen (VIB-KU Leuven, ULB) discovered that mitochondria also control a crucial event at the time of brain development—that is, how neural stem cells convert to nerve cells.
This cell fate switch is influenced by mitochondria during an accurate period that is twice as long in human beings when compared to that of mice. The seminal discoveries emphasize an unanticipated role of mitochondria that may help describe how human beings developed a larger brain during evolution and how defects in the mitochondria cause neurodevelopmental diseases.
The human brain consists of an unlimited number of remarkably varied neurons. They initially appear in the developing brain when stem cells cease to renew on their own and differentiate into a specific type of neuron.
This biological process, known as neurogenesis, is accurately controlled to form the massive complex structure, that is, the human brain. Scientists believed that minute variations in the way neural stem cells create neurons are at the origin of the significant increase in the complexity and size of the human brain.
To have a better understanding of this intricate process, Professor Pierre Vanderhaeghen (VIB-KU Leuven, ULB) and his collaborators analyzed the mitochondria, which are tiny organelles that supply energy to all cells in the body, such as the developing brain.
Diseases caused by defects in mitochondria lead to developmental problems in many organs, in particular the brain. We used to think that this was related to the crucial function of mitochondria to provide energy to the cells, but this is only part of the story: recent work in stem cells suggests that mitochondria have a direct influence on organ development. We have tested whether and how this could be the case in the brain.”
Pierre Vanderhaeghen, Study Corresponding Author and Professor, Department of Neurosciences, Katholieke University Leuven
Professor Vanderhaeghen is also a specialist in stem cell and developmental neurobiology.
Fission and fusion
Vanderhaeghen and his team explored whether and how the remodeling of mitochondria is combined with neuronal fate commitment at the time of neurogenesis.
Mitochondria are highly dynamic organelles, that can join together (fusion) or split up (fission), and we know these dynamics are associated with fate changes in various types of stem cells.”
Pierre Vanderhaeghen, Study Corresponding Author and Professor, Department of Neurosciences, Katholieke University Leuven
A new technique was developed by Ryohei Iwata, a postdoctoral researcher in the Vanderhaeghen laboratory, to observe the mitochondria more closely, as the neural stem cells are “caught in the act” to turn into neurons.
We found that shortly after stem cells divide, the mitochondria in daughter cells destined to self-renew will fuse, while those in daughter cells that become neurons show high levels of fission instead.”
Ryohei Iwata, Postdoctoral Researcher, Department of Neurosciences, Katholieke University Leuven
However, this was not a mere coincidence: the team could indeed demonstrate that while increased mitochondrial fission actually supports differentiation to a neuronal fate, mitochondrial fusion, following mitosis, redirects the daughter cells towards the self-renewal process.
Time window
Thus, the dynamics of mitochondria are significant to become a neuronal cell, but there is more.
Pierre Casimir, a PhD student in Professor Vanderhaeghen’s laboratory, stated, “We found that the influence of mitochondrial dynamics on cell fate choice is limited to a very specific time window, right after cell division. Interestingly, the restricted time window is twice as long in humans compared to mice.”
Professor Vanderhaeghen added, “Previous findings were primarily focused on fate decision of neural stem cells before they divide, but our data reveal that cell fate can be influenced for a much longer period, even after neural stem cell division.”
This may hold fascinating implications in the emerging domain of cell reprogramming, where investigators attempt to directly change non-neuronal cells into neuronal cells for therapeutic purposes, for example.
“Since this period of plasticity is much longer in human cells compared to mouse cells, it is tempting to speculate that it contributes to the increased self-renewal capacity of human progenitor cells, and thus to the uniquely developed brain and cognitive abilities of our species. It is fascinating to think that mitochondria, small organelles that have evolved in cells more than a billion years ago, might have contributed to the recent evolution of the human brain,” Professor Vanderhaeghen concluded.
Source:
Journal reference:
Iwata, R., et al. (2020) Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science. doi.org/10.1126/science.aba9760.