Leipzig University scientists, in association with collaborators from Germany, Italy, and the Netherlands, have extensively studied the behavior of tumor cells and the structure of tumor tissue, gaining a better understanding of mechanisms that could enhance diagnosis and treatment for cancer in the days to come.
Schematic representation of different types of movement of tumor tissue in the extracellular matrix. Image Credit: Peter Friedl, Stefano Zappen, Andreas Deutsch, Josef A. Käs, and Jürgen Lippoldt.
The team discovered that during the development of tumors, the movement of cells could change from collective and coordinated one, to a chaotic and individual behavior. The study results were recently published in the Nature Cell Biology journal.
The study was monitored by Professor Peter Friedl, a tumor biologist from Radboud University in Nijmegen, the Netherlands, in association with the research teams led by Professor Josef A. Käs from Leipzig University, Professor Andreas Deutsch from Dresden University of Technology (TU Dresden), and Professor Stefano Zapperi from the University of Milan.
The researchers analyzed biological modifications that are experienced by cells during the development of cancer. The most typical of these modifications is the breakdown of the epithelial adhesion molecule, known as E-cadherin. To put this in simpler terms, the cells turn out to be less “sticky.”
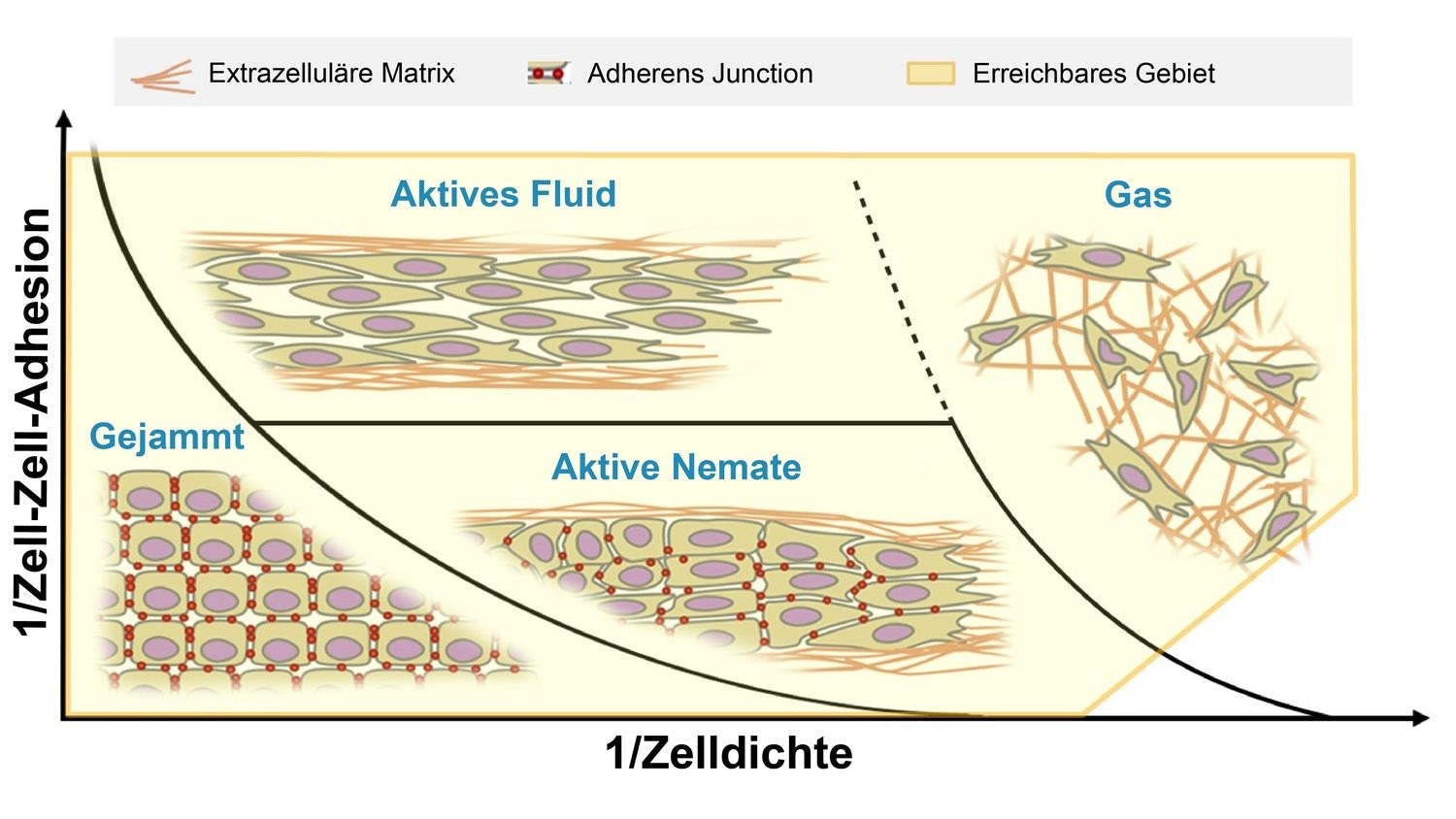
The team demonstrated that this kind of degradation is typically accompanied by a modification in the type of movement in the tissue. While the epithelial cells are “trapped” by their neighbors, cells that happen to be more cancerous can travel freely past others of their kind.
It has long been assumed that the reduction in cell ‘stickiness’ during tumour development increases the mobility of these cancer cells. Our international team was able to confirm this fundamental assumption and show that a dense environment can still hold cancer cells back.”
Josef A. Käs, Professor, Leipzig University
According to Käs, it is clear that the local environment strongly influences the invasion of tumors: cells that act separately can also migrate in clusters if this decreases the resistance of the surrounding tissue. The two types of cell movement resulted in metastases in the experiments conducted by the team.
A majority of the cancers are carcinomas that arise from epithelial tissue that encloses and separates the organs. The functions of epithelial tissues include support and protection. Cells in this epithelium, and remaining immobile under healthy conditions, serve as a typical example in new studies into “cell jamming”—a field which is being developed quickly.
Such an immobility is explained by the fact that the cells are in one another’s way—analogous to individual grains in a pile of sand, or cars in a traffic jam. Cancer cells should have the potential to travel through the body to metastasize. The phenotype of these cancer cells modifies during the development of tumor, shifting away from epithelial behavior.
In experiments performed on tumor cells isolated from patients, the team discovered that cancer cells proliferate in different ways in different settings—cells with an epithelial phenotype continued to stay in a closed network, where their movements were collective and coordinated.
Cells that are less “sticky” consequently became more cancerous, with their cohesion declining and movements growing more fluid. Individual and less “sticky” cells separated into the surrounding tissue.
This only happens if this tissue is not too dense. This movement is not coordinated, in step, as it would be in cells with an epithelial phenotype, but random and not coordinated with adjacent cells. In order to turn this understanding into an advantage for cancer patients, further research is needed to find out which migration method can lead to metastases under which circumstances.”
Jürgen Lippoldt, Doctoral Researcher, Leipzig University
Source:
Journal reference:
Ilina, O., et al. (2020) Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nature Cell Biology. doi.org/ 10.1038/s41556-020-0552-6.