For more than 15 years, ETH Professor Andreas Hierlemann and his team have been designing microelectrode-array chips that can be utilized to accurately stimulate nerve cells in cell cultures and to quantify the activity of electrical cell.
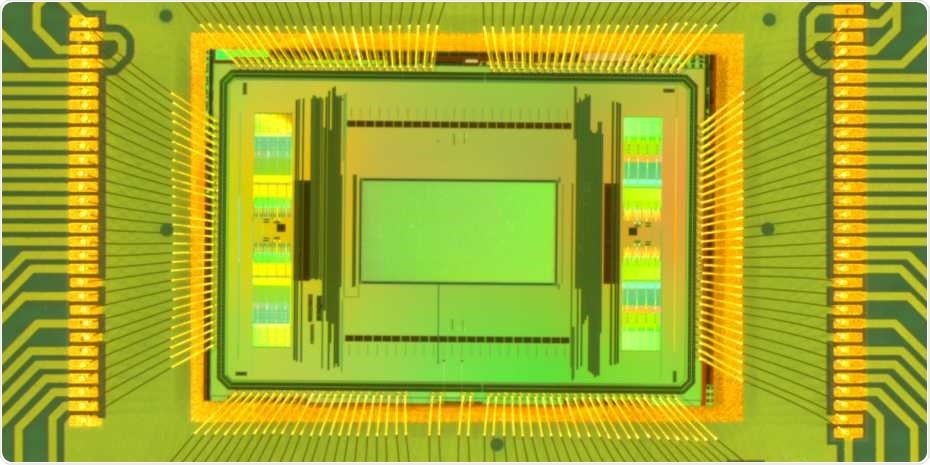
Close-up of the new dual-mode chip. The measurement area in the centre of the image (green) is 2 x 4 millimetres. Image Credit: ETH Zurich / Xinyue Yuan.
These advancements make it viable to grow nerve cells in cell-culture dishes and utilize the chips present at the base of the dish to study every individual cell in a connected nerve tissue more comprehensively.
Alternative approaches for performing these measurements have certain restrictions. Either they are quite time-consuming—because contact to every cell has to be separately established—or they involve the use of fluorescent dyes, which affect the cellular behavior and thus the result of the experiments.
Currently, scientists from Hierlemann’s team at the Department of Biosystems Science and Engineering of ETH Zurich in Basel, along with Urs Frey and his collaborators from the ETH spin-off MaxWell Biosystems, have designed a new generation of microelectrode-array chips.
These chips allow comprehensive recordings of significantly more electrodes than earlier systems, which provide opportunities for novel applications.
Stronger signal required
Similar to previous chip generations, the novel chips have about 20,000 microelectrodes in an area that measures 2 x 4 mm. To make sure that these electrodes detect the comparatively weak nerve impulses, the signals had to be amplified.
Examples of weak signals required by the team to detect include the nerve cells that are extracted from human pluripotent stem cells (iPS cells). Currently, these cells are employed in several cell-culture disease models. Another major reason to considerably amplify the signals is if the team wants to monitor the nerve impulses in axons, which are fine, thin fibrous extensions of a nerve cell.
But high-performance amplification electronics tend to take up space, which is why the earlier chip was able to concurrently amplify and read out signals from just 1,000 of the 20,000 electrodes. Even though the 1,000 electrodes can be randomly chosen, they must be determined before each measurement. This implies that detailed recordings can be made across just a fraction of the chip area at the time of measurement.
Background noise reduced
In the novel chip, the amplifiers are smaller, which allows the signals of all 20,000 electrodes to be amplified and quantified simultaneously. But these smaller amplifiers have higher noise levels. Hence, to ensure that the electrodes capture even the weakest nerve impulses, the team included a few of the larger and stronger amplifiers into the novel chips and used a nifty trick—they applied these strong amplifiers to detect the time points at which nerve impulses take place in the cell culture dish.
At these time points, the team can look for signals on the other electrodes, and taking the average of many consecutive signals, they can decrease the background noise. This process yields a clear picture of the signal activity over the whole area being quantified.
In the initial experiments, which were published in the Nature Communications journal, the researchers demonstrated their approach on human iPS-derived neuronal cells and also on retina pieces, neuronal spheroids, brain sections, and cardiac cells.
Application in drug development
Using the new chip, the researchers can create electrical images of both the cells and the extension of their axons, and they can even establish the speed at which a nerve impulse is transferred to the farthest reaches of the axons.
The previous generations of microelectrode array chips let us measure up to 50 nerve cells. With the new chip, we can perform detailed measurements of more than 1,000 cells in a culture all at once.”
Dr Andreas Hierlemann, Professor, ETH Zurich
Such detailed measurements are appropriate for examining the effects of medications, which means investigators can now perform studies and experiments with human cell cultures rather than depending on laboratory animals. Thus, the technique also helps decrease the number of animal experiments.
The ETH spin-off MaxWell Biosystems is already commercializing the prevailing microelectrode technology, which is being used worldwide by more than a hundred research teams in industry and at universities. Currently, the company is looking into a possible commercialization of the novel chip.
Source:
Journal reference:
Yuan, X., et al. (2020) Versatile live-cell activity analysis platform for characterization of neuronal dynamics at single-cell and network level. Nature Communications. doi.org/10.1038/s41467-020-18620-4.