Scientists from the University of Cologne have found that a modification in the DNA structure— specifically in the chromatin—plays a key role in the recovery stage following DNA damage. The key is a double occupation by a pair of methyl groups on histone H3 (H3K4me2)—the DNA packaging protein.
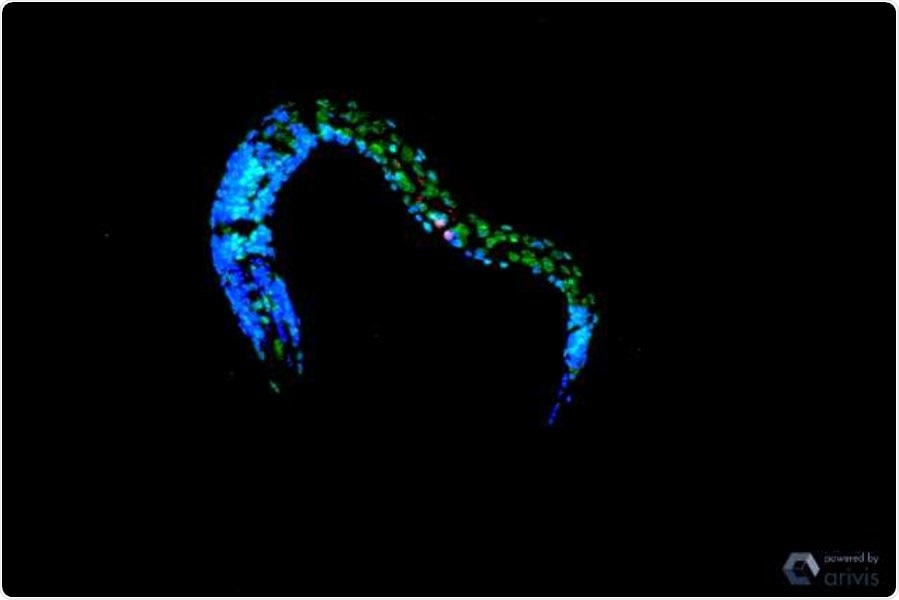
The image shows a confocal microscopy of C. elegans after UV irradiation. There are increased double occupations of the histones: H3K4me2 in green. Triple occupancy (H3K4me3) are stained red and nuclei are marked blue. Image Credit: Dr Siyao Wang.
The breakthrough was made by researchers under the guidance of Professor Björn Schumacher from the Cluster of Excellence for Aging Research CECAD, the Center for Molecular Medicine Cologne (CMMC), and the Institute for Genome Stability in Aging and Disease at the University of Cologne.
The specific modification allows the reactivation of genes and the production of proteins following damage—the cells recover their balance and so does the organism. The researchers identified the protective function of H3K4me2 during experiments with the nematode Caenorhabditis elegans. The research work was recently published in the Nature Structural & Molecular Biology journal.
The genome found in each human cell is impaired on a daily basis, for instance, in the skin by the Sun’s UV radiation. DNA damage leads to various diseases, like cancer, affects development, and also speeds up aging. Congenital malfunctions in the DNA repairing process can even result in extremely accelerated aging in rare hereditary disorders.
Thus, reconstruction and preservation processes are specifically significant to ensure development and sustain the function of tissues. DNA, which is rolled up on histones—the packaging proteins—similar to cable drums, is controlled by methyl groups. Different proteins account for placing the methyl groups on histones or for removing them. Moreover, the number of groups present on the packaging proteins influences the activity of genes and therefore the cell’s protein synthesis.
In experiments performed with the nematode, the team demonstrated that once the damaged DNA is repaired, a pair of methyl groups was increasingly detected on the DNA packages. The researchers also discovered that errors in positioning these two methyl groups on the histones (H3K4me2) expedited the damage-induced aging process, whereas increased position of this histone modification extends the lifespan following DNA damage.
By regulating the proteins that either remove or set these methyl groups, the resistance to DNA damage—and therefore the animals’ aging process—could be controlled.
Additional analysis of the function of these two methyl groups revealed that H3K4 enriched with two methyl groups after genome damage supports the cells in reinstating the balance following DNA damage.
Now that we know the exact changes in chromatin, we can use this to precisely limit the consequences of DNA damage. I hope that these findings will enable us to develop therapies for hereditary diseases characterized by developmental disorders and premature aging. Due to the fundamental importance of DNA damage in the aging process, such approaches could also counteract normal aging and prevent age-related diseases.”
Björn Schumacher, Professor, Cluster of Excellence for Aging Research CECAD, Center for Molecular Medicine Cologne
Source:
Journal reference:
Wang, S., et al. (2020) H3K4me2 regulates the recovery of protein biosynthesis and homeostasis following DNA damage. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-020-00513-1.