A novel study into the immune response of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected patients by carbohydrates was recently published on the bioRxiv* preprint server, indicating that many of the unusual symptoms of SARS-CoV-2 infections may be due to induced autoimmune responses.
Image Credit: Corona Borealis Studio/Shutterstock.com

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Patients frequently exhibit a wide range of symptoms across several organs besides the lungs, including the gastrointestinal tract and cardiovascular systems, in some cases lingering for many months following initial infection, while many develop only minor or no symptoms. SARS-CoV-2 has also been shown to induce the production of autoantibodies, usually observed in children who relapse with multi inflammatory syndrome and adults with severe symptoms or pre-existing neurological conditions.
Additionally, a large portion of SARS-CoV-2 patients report neurological symptoms such as loss of taste and smell, while most cerebrospinal fluid samples are absent of the virus. These potential long-term symptoms resemble those seen in autoimmune disorders, and the highly individual responses to infection exhibited by patients may in part be ascribed to differing immune responses and antibody generation.
Interestingly, some recent reports have identified a correlation between blood type and susceptibility to severe symptoms, the difference between blood types being distinguished by the glycans on the outer surface. Similarly, the presence of non-human glycan antibody anti-α-Gal, often involved in immune responses to pathogens bearing similar glycans, has been shown to predict good patient outcomes for SARS-CoV-2 infection.
What causes the immune response?
The surface of SARS-CoV-2 is coated with glycans, and the SARS-CoV-2 spike protein, in particular, is heavily glycosylated with a variety of O- and N-linked glycans.
Butler & Gildersleeve collected serum samples from 20 symptomatic SARS-CoV-2 patients and passed them through a carbohydrate antigen microarray containing a large variety of glycans. This allowed them to assess the affinity of any present biomolecules towards the glycan chains compared to a control group of healthy individuals. They found that symptomatic individuals serum had a large number of antibodies to human gangliosides, N-linked glycans, and self-glycans, in quantities uncommon even compared to other conditions such as cancer, exceeding even the levels seen in HIV patients.
The production of antibodies to self-glycans has been observed in certain other bacterial and viral infections, including the Zika virus and HIV. In the case of SARS-CoV-2, this most likely occurs through the commandeering of host glycosylation machinery or the direct incorporation of host glycans from the endoplasmic reticulum into the viral envelope, stimulating an immune response to the hosts own self-glycans.
Which antibodies were affected?
IgM was consistently detected at levels 2-4 times lower than in the control group, while IgG remained relatively consistent between groups. Interestingly, total IgM within the serum samples was found to be on average only 30% lower in the SARS-CoV-2 group than in control, indicating that the surface glycans of IgM are capable of recognition on the microarray were in some way compromised.
Anti-glycolipid antibody concentration was also significantly greater in the SARS-CoV-2 group, usually indicative of autoimmune disease. The specific antibody with elevated signals varied among patients, though in 70% of the SARS-CoV-2 group exhibited unusually high antibodies to at least one glycolipid.
Similarly, IgG signals to N-linked glycans were elevated compared to the control group that had little measurable signal. The authors highlight in particular an abundance of IgG signals to NGA4 and NGA3, but not to NGA2 or NGA3B, indicating a highly specific response to these glycan configurations. Similarly, over half of the SARS-CoV-2 patients showed high IgG signals for one or more oligomannose glycans, with more than a third of the patients for two.
N-acetyllactosamine is a carbohydrate structure found on many N- and O-linked glycans and glycolipids commonly throughout the human body. Several of the SARS-CoV-2 group were identified as having high IgM or IgG signals towards N-acetyllactosamine and several other self-glycans, which is particularly notable due to the total IgM or IgG to most other glycans being significantly lessened.
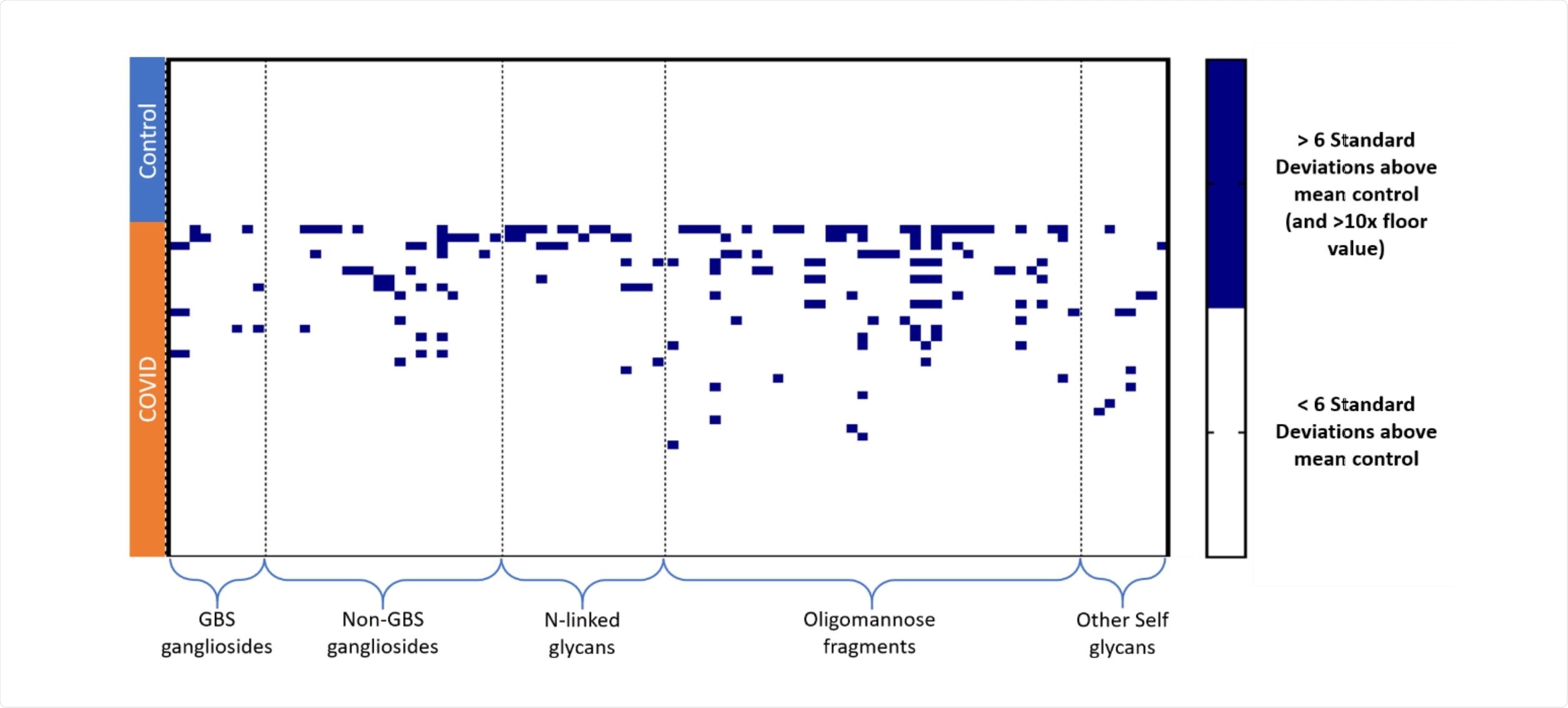
Image Credit: https://www.biorxiv.org/content/10.1101/2020.10.15.341479v1.full.pdf
Did antibodies recognize the SARS-CoV-2 spike protein?
Several monoclonal antibodies known to bind with oligomannose glycans were tested using an ELISA assay with the two subunits and binding domain of SARS-CoV-2, finding that anti-HIV antibody PGT126 and its similar cousins had the greatest affinity.
What affect will this have on treatment?
Treatment involving the administration of convalescent serum has received renewed interest during the pandemic, however, an overly aggressive application of neutralizing antibodies has been demonstrated to be detrimental in many cases. Aberrant immune responses are associated with excessive inflammation and severe respiratory trauma, and in recognition of this convalescent serum is frequently given alongside anti-inflammatory agents such as dexamethasone.
Currently, convalescent plasma is filtered to remove infectious diseases and certain other antibodies, however, given the evidence that antibodies are frequently involved in autoimmune dysfunction during SARS-CoV-2, it should be ensured that none of the antibodies discussed are present.
Additionally, future vaccines will need to account for potential complications involved in inducing autoantibodies, as both live-attenuated and inactivated virus vaccines usually still display the glycans on the surface.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
https://www.biorxiv.org/content/10.1101/2020.10.15.341479v1